��Ŀ����
����Ŀ�������£�NaOH��Һ�ζ����������(�������Ka1��12��10��2��Ka2��63��10��8)��Һ���ζ���������Һ����Ե��������仯������ͼ��ʾ������b��Ϊ��Ӧ�յ㡣���������������
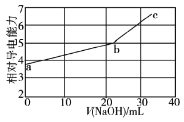
A.b��Ļ����ҺpH�����ܵ���7
B.����Ũ�Ⱥ������Ӱ����Һ�ĵ�������
C.C��Ļ����Һ�У�c(OH��)��c(Na��)��c(K��)
D.Na����SO32���ĵ�������֮�ʹ���HSO3���ĵ�������
���𰸡�C
��������
A����������ݿ�֪H2AΪ��Ԫ���ᣬb������ΪΪNa2S��K2S��Ϊǿ�������Σ���Һ�ʼ��ԣ���pH��7�������ܵ���7����A��ȷ��
B����Һ�ĵ�������ȡ���ڵ��Ũ�ȵĴ�С����ͼ���֪a��b��c����������ࡢŨ�Ȳ�ͬ����֪�����Һ�ĵ�������������Ũ�Ⱥ������йأ���B��ȷ��
C��c��NaOH��������n(NaOH)��n(KHS)����Һ�ʼ��ԣ���֪c(Na+)��c(K+)��c(OH-)����C����
D����ͼ���֪b�������Ũ�Ƚ�С��b�㵼�������ϴ�b�����Na+��S2-����֪Na+��S2-�ĵ�������֮�ʹ���HS-�ģ���D��ȷ��
�ʴ�ΪC��

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д� ����������ϵ�д�
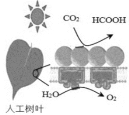
����������ϵ�д�����Ŀ��CO2�IJ����������������ʵ������������ŵ���Ҫ;��֮һ����ش�
(1)������̼�ĵ���ʽΪ__________��
(2)һ�����ڿ��������ö�����̼��ȡ����(HCOOH)��;����ͼ��ʾ��ͼ��������Ҫת����ʽΪ_____________ ��CO2��H2Oת��Ϊ����Ļ�ѧ����ʽΪ____________��
(3)Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���һ�������·�����Ӧ��CO2(g)+3H2(g)CH3OH(g)+H2O(g)��
�ٺ��������У��ܼӿ�÷�Ӧ���ʵ���_______��
a.�����¶� b.����ϵ�з����CH3OH c.�����Ч���� d.����ѹǿ
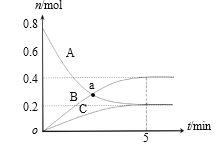
�������Ϊ2L���ܱ������У�����1molCO2��3molH2�����CO2�����ʵ�����ʱ��仯��ͼ��ʾ���ӷ�Ӧ��ʼ��5minĩ����H2Ũ�ȱ仯��ʾ��ƽ����Ӧ����________________��
t/min | 0 | 2 | 5 | 10 | 15 |
n(CO2)/mol | 1 | 0.75 | 0.5 | 0.25 | 0.25 |
������ͬ�¶ȡ����ݵ������£���˵���÷�Ӧ�Ѵ�ƽ��״̬����_______(�����)��
a.CO2��H2��CH3OH��H2O��Ũ�Ⱦ����ٸı仯
b.n(CO2):n(H2):n(CH3OH):n(H2O)=1:1:1:1
c.�����л��������ܶȲ���
d.v����(H2)=3v����(CH3OH)
e.��ϵѹǿ����
(4)����һЩ���ۼ��ļ������±���ʾ��
��ѧ�� | H-H | H-O | C=O | C-H | C-O |
����kJ/mol | 436 | 463 | 803 | 414 | 326 |
��ӦCO2(g)+3H2(g)CH3OH(g)+H2O(g)��______(���������������ų���) ������Ϊ_____kJ
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��гּ������������ԣ���
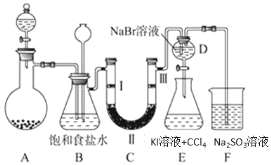
��1��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C���������������η�______
a | b | c | d | |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | ��ˮ�Ȼ��� | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��2�����װ��D��E��Ŀ���DZȽ��ȡ��塢�ⵥ�ʵ�������ǿ��������D�л���ͨ��һ����������ʱ�����Կ�����ɫ��Һ��Ϊ�Ȼ�ɫ����������װ��D��������Һ����װ��E�У����۲쵽���������²���Һ����ɫ����֤�����嵥�ʵ�������ǿ�ڵⵥ�ʣ�������ͬѧ�Ըý���������飬����������___________��
��3���ձ�F�е� ����������Һ��������β�������ʵ�鷽����֤β�����պ���Һ�к��� SO42-_____��
����Ŀ������ͼʾװ�ý���ʵ�飬���ܵó���Ӧ���۵��ǣ� ��
��Һ�� | ����� | ��Һ�� | ʵ����� |
| |
A | ϡ���� | Na2CO3 | Na2SiO3 | �ǽ����ԣ�S>C>Si | |
B | Ũ���� | KMnO4 | Na2S | �����ԣ�KMnO4>Cl2>S | |
C | ŨH2SO4 | Cu | ������ | ��Һ����� | |
D | Ũ��ˮ | CaO | AlCl3 | �����Ʊ�Al(OH)3 |
A. A B. B C. C D. D