��Ŀ����
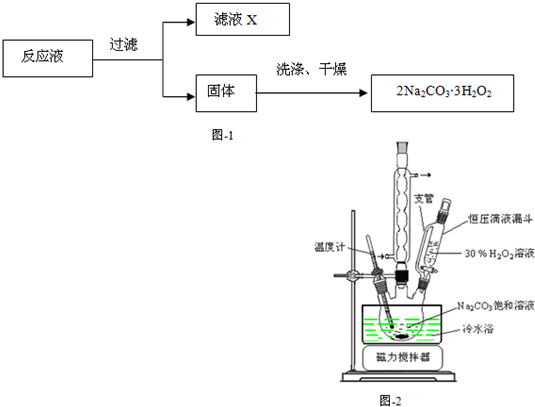
��̼���ƣ�2Na2CO3��3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʡ�����ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ��


��1����ѹ��Һ©����֧�ܵ������� ��
��2���Ʊ���̼���ƵĹؼ��� ��
��3��������ƹ�̼���Ƶ�ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��д������һ�ּ��ɣ��÷���ʽ��ʾ����________________________________��
��4��ij��ѧѧϰС��Ϊ�˶���̽�������Ӷ���������Ư���IJ���Ӱ�죬ȡ��Ư��100mL������25g FeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0��1mol/LNaOH��Һ��8��0mol/LNaOH��Һ������ʯ��ˮ��0��01mol/LKMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��ľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2�� ����2��������______________�� ����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۣ�
|
ʵ�鲽�� |
Ԥ����������� |
|
����������ͨ��ʢ��_______��________��ϴ��ƿ�У�________________________�� |
��________________________ ��________________________ ��________________________ |
��1��ʹҺ��˳�����£�1�֣�
��2�����Ʒ�Ӧ�¶ȣ�1�֣�
��3�� ��
�� ��1�֣�
��1�֣�
��4����CO2��O2 ��1�֣������������˳��ɵߵ���
�ڣ���6�֣�
|
ʵ����� |
Ԥ����������� |
|
����ʯ��ˮ��8��0mol��L-1NaOH��Һ�����������ǵ�ľ���������һ��ϴ��ƿ�ij��ڴ����������غŴ�Ϊ���÷ֵ㣬��1�֣��Լ�Ҫ��Ũ�ȣ���ֵ����λѡ����˳��ȷ�Ÿ��֣��� |
��������ʯ��ˮ������ǣ�ľ����ȼ�������1������ ��������ʯ��ˮ����ǣ�ľ����ȼ�������2������ ��������ʯ��ˮ����ǣ�ľ������ȼ�������3��������3�֣� |
��������
������������ʵ��װ��ͼ��֪��ѹ��Һ©����֧�ܵ�������ʹҺ̬˳�����£���ʵ��Ĺؼ��ǿ��Ʒ�Ӧ���¶ȣ������ƹ����У�������������ӣ�����CO32-����˫ˮ�⣬��4����������ɷֵļ��軹��CO2��O2 ���ֱ��ó���ʯ��ˮ�ʹ����ǵ�ľ�����飬���Ҽ�������ǰҪ��ȥ������̼�����������ʯ��ˮ������ǣ�ľ����ȼ�������1��������������ʯ��ˮ����ǣ�ľ����ȼ�������2��������������ʯ��ˮ����ǣ�ľ������ȼ�������3������
���㣺��ѧʵ�顣

| A��ʵ�����Ʊ���̼����ʱ�������ˮԡ���Ʒ�Ӧ�¶� | B����̼����ˮ��Һ�ʼ��ԣ�������Ưϴ��������ɱ���� | C����̼����Ӧ�ܷⱣ�棬�������䰵�� | D����̼���ƿ�ʹ���Ը��������Һ��ɫ�����ų�һ����ɫ���� |



 ��֪������Ӧ 2Na2CO3 ��aq��+3H2O2 ��aq��
��֪������Ӧ 2Na2CO3 ��aq��+3H2O2 ��aq��  2Na2CO3?3H2O2 ��s��
2Na2CO3?3H2O2 ��s��