��Ŀ����
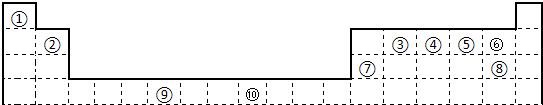
�±�Ϊ��ʽ���ڱ���һ���֣����е���ĸ������Ӧ��Ԫ�ء�

�Իش��������⣺
(1)a�ֱ���g��d�γɵĻ������зе�ϸߵ���___________���ѧʽ�����÷�����dԭ�ӵ��ӻ���ʽΪ
___________��
(2)c��d��e��fԪ�صĵ�һ������(I1)��С�����˳��Ϊ____________������ӦԪ�ط��ű�ʾ����
(3)�ɱ���Ԫ���γɵ�һ�������뵥��d3��Ϊ�ȵ����壬�仯ѧʽΪ_____________��
(4)Ԫ��h�Ķ��������ӵĻ�̬�����Ų�ʽΪ_______________
(5)�о����֣�ֻ��b��e��i����Ԫ�ص�һ�־�����г����ԣ��侧������ͼ��ʾ��bԭ�ӵ���λ��Ϊ
_________���þ���Ļ�ѧʽΪ_____________��
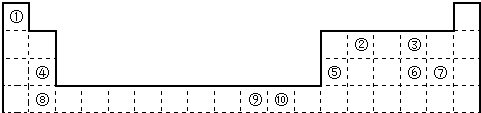
(1)a�ֱ���g��d�γɵĻ������зе�ϸߵ���___________���ѧʽ�����÷�����dԭ�ӵ��ӻ���ʽΪ
___________��
(2)c��d��e��fԪ�صĵ�һ������(I1)��С�����˳��Ϊ____________������ӦԪ�ط��ű�ʾ����
(3)�ɱ���Ԫ���γɵ�һ�������뵥��d3��Ϊ�ȵ����壬�仯ѧʽΪ_____________��
(4)Ԫ��h�Ķ��������ӵĻ�̬�����Ų�ʽΪ_______________
(5)�о����֣�ֻ��b��e��i����Ԫ�ص�һ�־�����г����ԣ��侧������ͼ��ʾ��bԭ�ӵ���λ��Ϊ
_________���þ���Ļ�ѧʽΪ_____________��

(6)�����е�һ��Ԫ���γɵĺ�����Ľṹ����ͼ��ʾ���Ҫ˵��������������ˮ��ԭ��_____________��

(1)H2O��sp3
(2)Al<Mg<O<N
(3)NO2-
(4)1s22s22p63s23p63d6
(5)6��MgNi3C
(6)HNO3��H2O��Ϊ���Է��ӣ�������������ԭ����HNO3������ˮ������HNO3�����е�-OH��H2O֮����γ������ʹHNO3��������ˮ
(2)Al<Mg<O<N
(3)NO2-
(4)1s22s22p63s23p63d6
(5)6��MgNi3C
(6)HNO3��H2O��Ϊ���Է��ӣ�������������ԭ����HNO3������ˮ������HNO3�����е�-OH��H2O֮����γ������ʹHNO3��������ˮ

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ