题目内容
已知:Fe2O3(s)+C(s)===CO2(g)+2Fe(s) ΔH=+234.14 kJ·mol-1
C(s)+O2(g)===CO2(g) ΔH=-393.5 kJ·mol-1
则2Fe(s)+O2(g)===Fe2O3(s)的ΔH是
C(s)+O2(g)===CO2(g) ΔH=-393.5 kJ·mol-1
则2Fe(s)+O2(g)===Fe2O3(s)的ΔH是
| A.-824.39 kJ·mol-1 | B.+ 627.6 kJ·mol-1 |
| C.-744.7 kJ·mol-1 | D.-169.4 kJ·mol-1 |
A
略

练习册系列答案
 举一反三同步巧讲精练系列答案
举一反三同步巧讲精练系列答案 口算与应用题卡系列答案
口算与应用题卡系列答案 名师点睛字词句段篇系列答案
名师点睛字词句段篇系列答案
相关题目
 CO2 (g)+ H2 (g)反应过程能量变化如右图所示,该反应为 反应(填“吸热”或“放热”),反应的热化学方程式为: 。
CO2 (g)+ H2 (g)反应过程能量变化如右图所示,该反应为 反应(填“吸热”或“放热”),反应的热化学方程式为: 。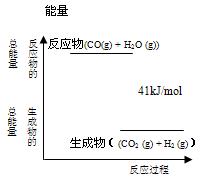
 Cu2O + H2↑。
Cu2O + H2↑。
 出N2。该制法的化学方程式为 ▲ 。
出N2。该制法的化学方程式为 ▲ 。 △H>0,水蒸气的浓度随时间t变化如下表所示。
△H>0,水蒸气的浓度随时间t变化如下表所示。