��Ŀ����
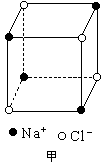
������й���ļ������Σ���������������ظ���λ��Ϊ������NaCl����ṹͼ����ͼ��
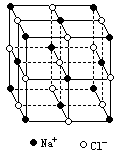
��֪![]() ���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xֵС��1����֪�����ܶȦ�Ϊ
���徧���ṹΪNaCl�ͣ����ھ���ȱ�ݣ�xֵС��1����֪�����ܶȦ�Ϊ![]() �������߳�Ϊ
�������߳�Ϊ![]() (��ԭ����Ϊ55.9����ԭ����Ϊ16)����
(��ԭ����Ϊ55.9����ԭ����Ϊ16)����
(1)![]() ��xֵ(��ȷ��0.01)Ϊ���٣�
��xֵ(��ȷ��0.01)Ϊ���٣�
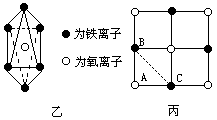
(2)�����е�Fe�ֱ�Ϊ![]() ��
��![]() ����
����![]() ��
��![]() ��������
�������У�![]() ��ռ����(��С����ʾ����ȷ��0.001)ΪΪ���٣�
��ռ����(��С����ʾ����ȷ��0.001)ΪΪ���٣�
(3)�˾��廯ѧʽΪ��
(4)Fe�ڴ˾�����ռ�ݿ�϶�ļ�����״Ϊ��(����![]() ��������ҵȾ����������Χ�ɵĿռ���״)��
��������ҵȾ����������Χ�ɵĿռ���״)��
(5)�ھ����У���Ԫ�ص����Ӽ���̾���Ϊ���٣�
������
|
(1)��NaCl����ṹ��֪��1��NaCl������8��С�����幹�ɣ�ÿ��С�������8�����ֱ���4��
��
�� ��֪Feԭ����Ϊ55.9����x=0.92 (2)�� 2y��3(0.92��y)=2 ����y=0.76(��)���� (3)���� (4)��ͼ�ҿ�֪����
��˴�ͼ�п�֪Fe�� (5)����Fe��Fe��̵ľ���Ϊ��(Fe��Fe)����
���ԣ���(Fe��Fe)= |



 2s
2s 3s
3s 4s
4s �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ�� 
 2s
2s 3s
3s 4s
4s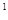 �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��