��Ŀ����
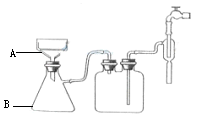
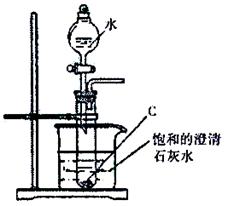
��10�֣���ʵ������ѧ��������ͼװ����ȡ����������

д���÷�Ӧ�Ļ�ѧ����ʽ______________________________��
�ش��������⣺
��1���ڴ��Թ��м���Ũ����3mL��������3mL��3 g�����Ҵ�4mL��2.7 g���������Լ�����ȷ������__________________________________��
��2��װ����ͨ�����ĵ���ֻ�ܲ嵽����̼������Һ��Һ���Ϸ�����������Һ�У�������__________�������ܵ�������_______________���������һ��װ��Ҳ��ʵ�������������ã������߲��ֻ�����װ�ü�ͼ��
��3���Թ����е�������_______ ���ɴ˿�֪��������������������_________________ ��
��4����ַ�Ӧ���Ƶ���������������_______________g��
��5����ҵ��ʯ���ѽ�������Ҫ�ɷ�Ϊԭ����ȡ���������������ķ�Ӧ���ͣ�����Ӧ˳����_________________________��

д���÷�Ӧ�Ļ�ѧ����ʽ______________________________��
�ش��������⣺
��1���ڴ��Թ��м���Ũ����3mL��������3mL��3 g�����Ҵ�4mL��2.7 g���������Լ�����ȷ������__________________________________��
��2��װ����ͨ�����ĵ���ֻ�ܲ嵽����̼������Һ��Һ���Ϸ�����������Һ�У�������__________�������ܵ�������_______________���������һ��װ��Ҳ��ʵ�������������ã������߲��ֻ�����װ�ü�ͼ��
��3���Թ����е�������_______ ���ɴ˿�֪��������������������_________________ ��
��4����ַ�Ӧ���Ƶ���������������_______________g��
��5����ҵ��ʯ���ѽ�������Ҫ�ɷ�Ϊԭ����ȡ���������������ķ�Ӧ���ͣ�����Ӧ˳����_________________________��
CH3COOH��CH3CH2OH  CH3COOC2H5��H2O��
CH3COOC2H5��H2O��
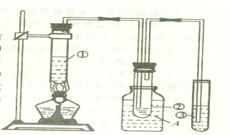
��1�����Թ��м�4mL�Ҵ���Ȼ���ҡ���Թܱ���������3mLŨ�����3mL ���ᡣ��ֻҪ���Ũ�����ҡ���Թܱ��������Լ����ɣ�
��2����ֹ���� ����������

��3������̼������Һ��Һ������������״Һ����������ŵ���ζ��
��������������ˮ���ܶȱ�ˮС���ӷ�����е�ͣ���
��4����4.4g
��5���ӳɷ�Ӧ��������Ӧ��������Ӧ
 CH3COOC2H5��H2O��
CH3COOC2H5��H2O����1�����Թ��м�4mL�Ҵ���Ȼ���ҡ���Թܱ���������3mLŨ�����3mL ���ᡣ��ֻҪ���Ũ�����ҡ���Թܱ��������Լ����ɣ�
��2����ֹ���� ����������

��3������̼������Һ��Һ������������״Һ����������ŵ���ζ��
��������������ˮ���ܶȱ�ˮС���ӷ�����е�ͣ���
��4����4.4g
��5���ӳɷ�Ӧ��������Ӧ��������Ӧ
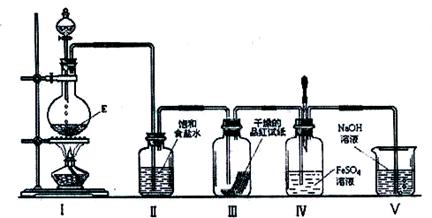
ʵ������ȡ����������������Ҵ����ã�����ʽΪCH3COOH��CH3CH2OH  CH3COOC2H5��H2O��
CH3COOC2H5��H2O��
��1��Ũ��������ˮ�ų��������ȣ����ܶȴ���ˮ�ģ�����Ӧ�������Թ��м�4mL�Ҵ���Ȼ���ҡ���Թܱ���������3mLŨ�����3mL ���ᡣ
��2���ӷ�������������Ҵ����Ǻ�ˮ���ܵģ����ֱ�Ӳ�����Һ�У����������������������������á�Ҫ�����ϵ����ã�������ֱ�ĸ���ܼ��ɣ���ͼ��ʾ�����𰸣���
��3����������������ˮ�������ڱ���̼������Һ��Һ������������״Һ��������ҿ��ŵ���ζ��
��4�������������ݿ�ʼ��������������������Ӧ����3g��2.3g��0.9g��4.4g������Ӧ�����淴Ӧ������ʵ�����ɵ���������С��4.4g��
��5���ѽ����к�����ϩ����ϩ��ˮ�ӳɼ��õ��Ҵ����Ҵ��������õ����ᣬ������Ҵ���������������������
 CH3COOC2H5��H2O��
CH3COOC2H5��H2O����1��Ũ��������ˮ�ų��������ȣ����ܶȴ���ˮ�ģ�����Ӧ�������Թ��м�4mL�Ҵ���Ȼ���ҡ���Թܱ���������3mLŨ�����3mL ���ᡣ
��2���ӷ�������������Ҵ����Ǻ�ˮ���ܵģ����ֱ�Ӳ�����Һ�У����������������������������á�Ҫ�����ϵ����ã�������ֱ�ĸ���ܼ��ɣ���ͼ��ʾ�����𰸣���
��3����������������ˮ�������ڱ���̼������Һ��Һ������������״Һ��������ҿ��ŵ���ζ��
��4�������������ݿ�ʼ��������������������Ӧ����3g��2.3g��0.9g��4.4g������Ӧ�����淴Ӧ������ʵ�����ɵ���������С��4.4g��
��5���ѽ����к�����ϩ����ϩ��ˮ�ӳɼ��õ��Ҵ����Ҵ��������õ����ᣬ������Ҵ���������������������

��ϰ��ϵ�д�
�����Ŀ

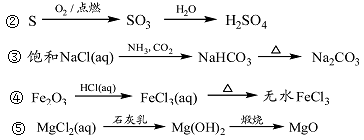
 CH3CH2Br+NaHSO4 +H2O��
CH3CH2Br+NaHSO4 +H2O��
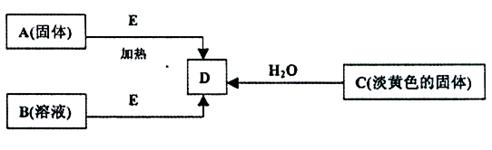
 ��
��