ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΥ’Κœœψ¥ΦΩ…“‘”ΟΉς ≥”ΟœψΨΪΘ§ΤδΫαΙΙΦρ Ϋ»γΆΦ1Υυ ΨΘ°

“―÷ΣΘΚR-CH=CH2 ![]() R-CH2CH2OH
R-CH2CH2OH
![]() “Μ
“Μ![]() Υ’Κœœψ¥ΦΒΡΖ÷Ή” ΫΈΣ ______ Θ§Υϋ≤ΜΡήΖΔ…ζΒΡ”–ΜζΖ¥”Πάύ–Ά”–
Υ’Κœœψ¥ΦΒΡΖ÷Ή” ΫΈΣ ______ Θ§Υϋ≤ΜΡήΖΔ…ζΒΡ”–ΜζΖ¥”Πάύ–Ά”–![]() Χν ΐΉ÷–ρΚ≈
Χν ΐΉ÷–ρΚ≈![]() ______ Θ°
______ Θ°
![]() »Γ¥ζΖ¥”Π
»Γ¥ζΖ¥”Π![]() Φ”≥…Ζ¥”Π
Φ”≥…Ζ¥”Π![]() œϊ»ΞΖ¥”Π
œϊ»ΞΖ¥”Π![]() Φ”ΨέΖ¥”Π
Φ”ΨέΖ¥”Π![]() ―θΜ·Ζ¥”Π
―θΜ·Ζ¥”Π![]() Υ°ΫβΖ¥”ΠΘ§
Υ°ΫβΖ¥”ΠΘ§
![]() Εΰ
Εΰ![]() ”–ΜζΈο±ϊ «“Μ÷÷œψΝœΘ§ΤδΚœ≥…¬ΖœΏ»γΆΦ
”–ΜζΈο±ϊ «“Μ÷÷œψΝœΘ§ΤδΚœ≥…¬ΖœΏ»γΆΦ![]() Τδ÷–ΦΉΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΆ®Ιΐ÷ ΤΉΖ®≤βΒΟΈΣ88Θ§ΥϋΒΡΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ÷Μ”–»ΐΉιΖεΘΜ““”κΥ’Κœœψ¥ΦΜΞΈΣΆ§œΒΈοΘ°
Τδ÷–ΦΉΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΆ®Ιΐ÷ ΤΉΖ®≤βΒΟΈΣ88Θ§ΥϋΒΡΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ÷Μ”–»ΐΉιΖεΘΜ““”κΥ’Κœœψ¥ΦΜΞΈΣΆ§œΒΈοΘ°
![]() Α¥’’œΒΆ≥ΟϋΟϊΖ®Θ§AΒΡΟϊ≥Τ « ______ Θ°
Α¥’’œΒΆ≥ΟϋΟϊΖ®Θ§AΒΡΟϊ≥Τ « ______ Θ°
![]() ”κ–¬÷Τ
”κ–¬÷Τ![]() –ϋΉ«“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ______ Θ°
–ϋΉ«“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ______ Θ°
![]() ±ϊ÷–Κ§”–ΝΫΗω
±ϊ÷–Κ§”–ΝΫΗω![]() Θ§DΩ…ΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬1molD”κ2mol
Θ§DΩ…ΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬1molD”κ2mol![]() Ω…“‘Ζ¥”Π…ζ≥…““Θ§‘ρDΒΡΫαΙΙΦρ ΫΈΣ ______ Θ°
Ω…“‘Ζ¥”Π…ζ≥…““Θ§‘ρDΒΡΫαΙΙΦρ ΫΈΣ ______ Θ°
![]() ΦΉ”κ““Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ______ Θ°
ΦΉ”κ““Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ ______ Θ°
![]() ±ΫΜΖ…œ”–3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈Θ§«“œ‘»θΥα–‘ΒΡ““ΒΡΆ§Ζ÷“λΙΙΧεΙ≤”– _____ ÷÷Θ§Τδ÷–3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈ΜΞ≤ΜœύΝΎΒΡ”–ΜζΈοΫαΙΙΦρ ΫΈΣ ______ Θ°
±ΫΜΖ…œ”–3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈Θ§«“œ‘»θΥα–‘ΒΡ““ΒΡΆ§Ζ÷“λΙΙΧεΙ≤”– _____ ÷÷Θ§Τδ÷–3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈ΜΞ≤ΜœύΝΎΒΡ”–ΜζΈοΫαΙΙΦρ ΫΈΣ ______ Θ°
ΓΨ¥πΑΗΓΩ![]()
![]()
![]() ΦΉΜυ±ϊœ© (CH3)2CHCHO+2Cu(OH)2
ΦΉΜυ±ϊœ© (CH3)2CHCHO+2Cu(OH)2 ![]() (CH3)2CHCOOH+Cu2OΓΐ+2H2O
(CH3)2CHCOOH+Cu2OΓΐ+2H2O ![]()
![]()
![]()
![]()
![]()
![]() 10
10 
ΓΨΫβΈωΓΩ
(“Μ)ΗυΨίΥ’Κœœψ¥ΦΒΡΫαΙΙΦρ ΫΖ÷ΈωΫβ¥πΘΜ
(Εΰ)““”κΥ’Κœœψ¥ΦΜΞΈΣΆ§œΒΈοΘ§““ΈΣ¥ΦΘ§ΗυΨίΝς≥ΧΆΦΘ§ΦΉΈΣτ»ΥαΘ§ΦΉΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΆ®Ιΐ÷ ΤΉΖ®≤βΒΟΈΣ88Θ§”…±ϊΒΡΖ÷Ή” ΫΩ…÷ΣΘ§ΦΉΒΡΖ÷Ή” ΫΈΣ![]() Θ§ΦΉΒΡΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ÷Μ”–»ΐΉιΖεΘ§‘ρΦΉΈΣ
Θ§ΦΉΒΡΚΥ¥≈Ι≤’ώ«βΤΉœ‘ Ψ÷Μ”–»ΐΉιΖεΘ§‘ρΦΉΈΣ![]() ΦΉΜυ±ϊΥαΘ§ΫαΚœΚœ≥…ΉΣΜ·ΆΦ÷–ΒΡΖ¥”ΠΧθΦΰΩ…÷ΣΘ§AΈΣ
ΦΉΜυ±ϊΥαΘ§ΫαΚœΚœ≥…ΉΣΜ·ΆΦ÷–ΒΡΖ¥”ΠΧθΦΰΩ…÷ΣΘ§AΈΣ![]() ΦΉΜυ±ϊœ©Θ§BΈΣ
ΦΉΜυ±ϊœ©Θ§BΈΣ![]() ΦΉΜυ±ϊ¥ΦΘ§CΈΣ
ΦΉΜυ±ϊ¥ΦΘ§CΈΣ![]() ΦΉΜυ±ϊ»©Θ§ΦΉ”κ““Ζ¥”ΠθΞΜ·…ζ≥…±ϊ
ΦΉΜυ±ϊ»©Θ§ΦΉ”κ““Ζ¥”ΠθΞΜ·…ζ≥…±ϊ![]() Θ§‘ρ““ΈΣ
Θ§‘ρ““ΈΣ![]() Θ§(3)÷–±ϊ÷–Κ§”–ΝΫΗω
Θ§(3)÷–±ϊ÷–Κ§”–ΝΫΗω![]() Θ§DΩ…ΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬
Θ§DΩ…ΖΔ…ζ“χΨΒΖ¥”ΠΘ§‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬![]() ”κ
”κ![]() Ω…“‘Ζ¥”Π…ζ≥…““Θ§Υυ“‘DΈΣ
Ω…“‘Ζ¥”Π…ζ≥…““Θ§Υυ“‘DΈΣ![]() Θ§‘ρDΒΡΫαΙΙΦρ ΫΈΣ
Θ§‘ρDΒΡΫαΙΙΦρ ΫΈΣ![]() Θ§““ΈΣ
Θ§““ΈΣ![]() Θ§‘ρ±ϊΈΣ
Θ§‘ρ±ϊΈΣ![]() ΓΘΨί¥ΥΖ÷ΈωΫβ¥πΓΘ
ΓΘΨί¥ΥΖ÷ΈωΫβ¥πΓΘ
(“Μ)(1)Υ’Κœœψ¥Φ( )ΒΡΖ÷Ή” ΫΈΣ
)ΒΡΖ÷Ή” ΫΈΣ![]() Θ§ΫαΙΙ÷–Κ§”–
Θ§ΫαΙΙ÷–Κ§”–![]() ΚΆ±ΫΜΖΘ§ ΡήΖΔ…ζ»Γ¥ζΓΔΦ”≥…ΓΔ―θΜ·ΓΔœϊ»ΞΖ¥”ΠΘ§Εχ≤ΜΡήΖΔ…ζΥ°ΫβΓΔΦ”ΨέΖ¥”ΠΘ§ Ι ¥πΑΗΈΣΘΚ
ΚΆ±ΫΜΖΘ§ ΡήΖΔ…ζ»Γ¥ζΓΔΦ”≥…ΓΔ―θΜ·ΓΔœϊ»ΞΖ¥”ΠΘ§Εχ≤ΜΡήΖΔ…ζΥ°ΫβΓΔΦ”ΨέΖ¥”ΠΘ§ Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ![]() ΘΜ
ΘΜ
(Εΰ)(2)”……œ ωΖ÷ΈωΩ…÷ΣΘ§AΈΣ![]() ΦΉΜυ±ϊœ©
ΦΉΜυ±ϊœ©![]() ΜρΦΉΜυ±ϊœ©
ΜρΦΉΜυ±ϊœ©![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΦΉΜυ±ϊœ©ΘΜ
ΦΉΜυ±ϊœ©ΘΜ
(3)C”κ–¬÷Τ![]() –ϋΉ«“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ(CH3)2CHCHO+2Cu(OH)2
–ϋΉ«“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ(CH3)2CHCHO+2Cu(OH)2 ![]() (CH3)2CHCOOH+Cu2OΓΐ+2H2O Θ§ Ι ¥πΑΗΈΣΘΚ(CH3)2CHCHO+2Cu(OH)2
(CH3)2CHCOOH+Cu2OΓΐ+2H2O Θ§ Ι ¥πΑΗΈΣΘΚ(CH3)2CHCHO+2Cu(OH)2 ![]() (CH3)2CHCOOH+Cu2OΓΐ+2H2OΘΜ
(CH3)2CHCOOH+Cu2OΓΐ+2H2OΘΜ
(4)ΦΉ”κ““Ζ¥”ΠθΞΜ·…ζ≥…±ϊ![]() Θ§±ϊ÷–Κ§”–ΝΫΗω
Θ§±ϊ÷–Κ§”–ΝΫΗω![]() Θ§‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬1molD”κ
Θ§‘Ύ¥ΏΜ·ΦΝ¥φ‘Ύœ¬1molD”κ![]() Ω…“‘Ζ¥”Π…ζ≥…““Θ§D÷–≤ΜΚ§ΦΉΜυΘ§Υυ“‘DΈΣDΈΣ
Ω…“‘Ζ¥”Π…ζ≥…““Θ§D÷–≤ΜΚ§ΦΉΜυΘ§Υυ“‘DΈΣDΈΣ![]() Θ§‘ρDΒΡΫαΙΙΦρ ΫΈΣ
Θ§‘ρDΒΡΫαΙΙΦρ ΫΈΣ![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ
(5)ΦΉ”κ““Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ![]()
![]()
![]()
![]()
![]() Θ§Ι ¥πΑΗΈΣΘΚ
Θ§Ι ¥πΑΗΈΣΘΚ![]()
![]()
![]()
![]()
![]() ΘΜ
ΘΜ
(6)““ΈΣ![]() Θ§ΫαΙΙΦρ ΫΈΣ
Θ§ΫαΙΙΦρ ΫΈΣ![]() Θ§ΤδΆ§Ζ÷“λΙΙΧεΖϊΚœΘΚ±ΫΜΖ…œ”–3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈Θ§œ‘»θΥα–‘Θ§‘ρ≤ύΝ¥ΈΣ
Θ§ΤδΆ§Ζ÷“λΙΙΧεΖϊΚœΘΚ±ΫΜΖ…œ”–3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈Θ§œ‘»θΥα–‘Θ§‘ρ≤ύΝ¥ΈΣ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() Θ§»τ
Θ§»τ![]() ΓΔ
ΓΔ![]() œύΝΎΘ§
œύΝΎΘ§![]() ”–4÷÷ΈΜ÷ΟΘ§»τ»τ
”–4÷÷ΈΜ÷ΟΘ§»τ»τ![]() ΓΔ
ΓΔ![]() œύΦδΘ§
œύΦδΘ§![]() ”–4÷÷ΈΜ÷ΟΘ§»τ»τ
”–4÷÷ΈΜ÷ΟΘ§»τ»τ![]() ΓΔ
ΓΔ![]() œύΕ‘Θ§
œύΕ‘Θ§![]() ”–2÷÷ΈΜ÷ΟΘ§Ι Ι≤”–10÷÷Θ§Τδ÷–±ΫΜΖ…œ3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈ΜΞ≤ΜœύΝΎΘ§‘ρΗΟΆ§Ζ÷“λΙΙΧεΈΣ
”–2÷÷ΈΜ÷ΟΘ§Ι Ι≤”–10÷÷Θ§Τδ÷–±ΫΜΖ…œ3Ηω»Γ¥ζΜυΜρΙΌΡήΆ≈ΜΞ≤ΜœύΝΎΘ§‘ρΗΟΆ§Ζ÷“λΙΙΧεΈΣ Θ§Ι ¥πΑΗΈΣΘΚ10ΘΜ
Θ§Ι ¥πΑΗΈΣΘΚ10ΘΜ ΓΘ
ΓΘ

ΓΨΧβΡΩΓΩΦΉ¥ΦΘ®CH3OHΘ©ΚΆΕΰΦΉΟ―Θ®CH3OCH3Θ©±Μ≥ΤΈΣ21 άΦΆΒΡ–¬–Ά»ΦΝœΘ§ΨΏ”–«εΫύΓΔΗΏ–ßΒ»–‘ΡήΓΘ
Θ®1Θ©CO2Ω…”Ο”ΎΚœ≥…ΕΰΦΉΟ―Θ®CH3OCH3Θ©Θ§”–ΙΊΖ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ»γœ¬ΘΚ
CO2(g) + 3H2(g)ΘΫCH3OH(g) + H2O(g) ΓςH=Θ≠49.0 kJΓΛmol-1
2CH3OH(g)ΘΫCH3OCH3(g) + H2O(g) ΓςH=Θ≠23.5 kJΓΛmol-1
H2O(l)ΘΫH2O(g) ΓςH= + 44 kJΓΛmol-1
‘ρCO2”κH2Ζ¥”ΠΚœ≥…ΕΰΦΉΟ―…ζ≥…“ΚΧ§Υ°ΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚ____________________ΓΘ
Θ®2Θ©ΙΛ“Β…œΚœ≥…ΦΉ¥ΦΒΡΖ¥”ΠΘΚCO(g)ΘΪ2H2(g)![]() CH3OH(g) ΓςH=Θ≠90.8 kJΓΛmol-1ΓΘ œ¬Ν–≤ΜΡήΥΒΟςΗΟΖ¥”Π‘ΎΚψΈ¬Κψ»ίΧθΦΰœ¬“―¥οΜ·―ßΤΫΚβΉ¥Χ§ΒΡ «___________
CH3OH(g) ΓςH=Θ≠90.8 kJΓΛmol-1ΓΘ œ¬Ν–≤ΜΡήΥΒΟςΗΟΖ¥”Π‘ΎΚψΈ¬Κψ»ίΧθΦΰœ¬“―¥οΜ·―ßΤΫΚβΉ¥Χ§ΒΡ «___________
AΘ°v’ΐ(H2) = 2vΡφ(CH3OH) BΘ°n(CO):n(H2):n(CH3OH)=1:2:1
CΘ°ΜλΚœΤχΧεΒΡΟήΕ»≤Μ±δ DΘ°ΜλΚœΤχΧεΒΡΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩ≤Μ±δ EΘ°»ίΤςΒΡ―Ι«Ω≤Μ±δ
Θ®3Θ©»τΖ¥”Π2CH3OH(g) ![]() CH3OCH3(g) + H2O(g)‘ΎΡ≥Έ¬Ε»œ¬ΒΡΜ·―ßΤΫΚβ≥Θ ΐΈΣ400Θ§¥ΥΈ¬Ε»œ¬Θ§‘ΎΟή±’»ίΤς÷–Φ”»κ“ΜΕ®ΝΩΦΉ¥ΦΘ§Ζ¥”ΠΫχ––ΒΫΡ≥ ±ΩΧΘ§≤βΒΟΗςΈο÷ ΒΡ≈®Ε»»γœ¬±μΥυ ΨΘΚ
CH3OCH3(g) + H2O(g)‘ΎΡ≥Έ¬Ε»œ¬ΒΡΜ·―ßΤΫΚβ≥Θ ΐΈΣ400Θ§¥ΥΈ¬Ε»œ¬Θ§‘ΎΟή±’»ίΤς÷–Φ”»κ“ΜΕ®ΝΩΦΉ¥ΦΘ§Ζ¥”ΠΫχ––ΒΫΡ≥ ±ΩΧΘ§≤βΒΟΗςΈο÷ ΒΡ≈®Ε»»γœ¬±μΥυ ΨΘΚ
Έο÷ | CH3OH(g) | CH3OCH3(g) | H2O(g) |
≈®Ε»Θ®molΓΛL-1Θ© | 0.44 | 0.60 | 0.60 |
ΔΌ±»ΫœΗΟ ±ΩΧ’ΐΓΔΡφΖ¥”ΠΥΌ¬ ΒΡ¥σ–ΓΘΚv(’ΐ)_____v(Ρφ)Θ®ΧνΓΑ>Γ±ΓΔΓΑ<Γ±ΜρΓΑ=Γ±Θ©ΓΘ
ΔΎ»τΦ”»κΦΉ¥ΦΚσΘ§Ψ≠10 minΖ¥”Π¥οΒΫΤΫΚβΘ§‘ρΤΫΚβΚσc(CH3OH)=______________Θ§
ΗΟ ±ΦδΡΎΖ¥”ΠΥΌ¬ v(CH3OCH3)=_____________ΓΘ
Θ®4Θ©άϊ”ΟΕΰΦΉΟ―Θ®CH3OCH3Θ©…ηΦΤ“ΜΗω»ΦΝœΒγ≥ΊΘ§”ΟKOH»ή“ΚΉςΒγΫβ÷ »ή“ΚΘ§ ·ΡΪΉωΒγΦΪΘ§ΗΟΒγ≥ΊΗΚΦΪΒγΦΪΖ¥”Π ΫΈΣ___________________________ΓΘ“‘¥Υ»ΦΝœΒγ≥ΊΉςΈΣΆβΫ”Βγ‘¥Α¥»γΆΦΥυ ΨΒγΫβΝρΥαΆ≠»ή“ΚΘ§»γΙϊΤπ Φ ± Δ”–1000mLpH=5ΒΡΝρΥαΆ≠»ή“ΚΘ®25ΓφΘ§CuSO4ΉψΝΩΘ©Θ§“ΜΕΈ ±ΦδΚσ»ή“ΚΒΡpH±δΈΣ1Θ§»τ“Σ Ι»ή“ΚΜ÷Η¥ΒΫΤπ Φ≈®Ε»Θ®Έ¬Ε»≤Μ±δΘ§Κω¬‘»ή“ΚΧεΜΐΒΡ±δΜ·Θ©Θ§Ω…œρ»ή“Κ÷–Φ”»κ______Τδ÷ ΝΩ‘ΦΈΣ_____gΓΘ

ΓΨΧβΡΩΓΩAΓΔBΓΔCΓΔDΓΔEΨυΈΣΕΧ÷ήΤΎ‘ΣΥΊΘ§‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΘ§«κΗυΨί±μ÷––≈œΔΜΊ¥πœ¬Ν–Έ ΧβΘΚ
‘ΣΥΊ | ‘ΣΥΊ–‘÷ ΜρΫαΙΙ |
A | ΉνΆβ≤ψΒγΉ” ΐ «ΤδΡΎ≤ψΒγΉ” ΐΒΡ2±Ε |
B | B‘ΣΥΊΒΡΒΞ÷ ‘ΎΩ’Τχ÷–Κ§ΝΩΉνΕύ |
C | C‘ΣΥΊ‘ΎΒΊΩ«÷–Κ§ΝΩΉνΕύ |
D | D‘ΣΥΊ‘ΎΆ§÷ήΤΎ÷–Ϋπ τ–‘Ήν«Ω |
E | ≥ΘΈ¬≥Θ―Ιœ¬Θ§E‘ΣΥΊ–Έ≥…ΒΡΒΞ÷ «Β≠ΜΤ…ΪΙΧΧεΘ§≥Θ‘ΎΜπ…ΫΩΎΗΫΫϋ≥ΝΜΐ |
Θ®1Θ©E‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡΈΜ÷Ο___Θ°
Θ®2Θ©BΉνΦρΒΞΤχΧ§«βΜ·ΈοΒΡΒγΉ” Ϋ___Θ§ τ”Ύ____Μ·ΚœΈοΘ®ΧνΓΑάκΉ”Γ±ΜρΓΑΙ≤ΦέΓ±Θ©ΘΜDΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΒγΉ” Ϋ___Θ§ΥυΚ§Μ·―ßΦϋάύ–ΆΘΚ___Θ°
Θ®3Θ©BΓΔCΓΔDΓΔEΦρΒΞάκΉ”ΑκΨΕ”…¥σΒΫ–ΓΥ≥–ρΈΣΘΚ ____Θ®ΧνάκΉ”ΖϊΚ≈Θ©Θ°
Θ®4Θ©”ΟΒγΉ” Ϋ±μ ΨEΒΡ«βΜ·ΈοΒΡ–Έ≥…Ιΐ≥Χ_____Θ°
Θ®5Θ©”…AΓΔBΓΔC”κ«β‘ΣΥΊΉι≥…ΒΡ“Μ÷÷≥ΘΦϊΥα Ϋ―Έ”κΙΐΝΩDΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ___Θ°
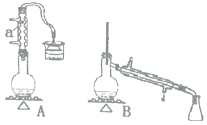
ΓΨΧβΡΩΓΩ Β―ι “”ΟΦ”»»1“ΜΕΓ¥ΦΓΔ≈®H2SO4ΚΆδεΜ·ΡΤΜλΚœΈοΒΡΖΫΖ®ά¥÷Τ±Η1“ΜδεΕΓΆιΘ§…ηΦΤΝΥ»γΆΦΥυ ΨΒΡ Β―ιΉΑ÷Ο![]() Τδ÷–ΒΡΦ–≥÷“«Τς“― Γ¬‘
Τδ÷–ΒΡΦ–≥÷“«Τς“― Γ¬‘![]() ΓΘ
ΓΘ
“―÷ΣΘΚH2SO4ΘΪNaBr=NaHSO4ΘΪHBrΘ§ H2SO4Θ®≈®Θ©ΘΪ2HBr=Br2ΘΪSO2ΓϋΘΪ2H2O
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)“«ΤςaΒΡΟϊ≥ΤΈΣ______ΓΘ
(2)÷Τ±Η≤ΌΉς÷–Θ§Φ”»κΒΡ≈®ΝρΥα ¬œ»“ΣΫχ––œΓ ΆΘ§ΤδΡΩΒΡ «______![]() Χν―ΓœνΉ÷ΡΗ
Χν―ΓœνΉ÷ΡΗ![]() ΓΘ
ΓΘ
![]() Φθ…ΌΗ±≤ζΈοœ©ΚΆΟ―ΒΡ…ζ≥…
Φθ…ΌΗ±≤ζΈοœ©ΚΆΟ―ΒΡ…ζ≥…![]() Φθ…Ό
Φθ…Ό![]() ΒΡ…ζ≥…
ΒΡ…ζ≥…![]() Υ° «Ζ¥”ΠΒΡ¥ΏΜ·ΦΝ
Υ° «Ζ¥”ΠΒΡ¥ΏΜ·ΦΝ
(3)–¥≥ω¥Υ Β―ι÷Τ1“ΜδεΕΓΆιΒΡΉήΜ·―ßΖΫ≥Χ Ϋ______ΓΘ
(4)”–Ά§―ßΡβΆ®ΙΐΚλΆβΙβΤΉ“«ΦχΕ®ΥυΒΟ≤ζΈο÷– «ΖώΚ§”–ΓΑ![]() Γ±Θ§ά¥»ΖΕ®Η±≤ζΈο÷– «Ζώ¥φ‘ΎΕΓΟ―
Γ±Θ§ά¥»ΖΕ®Η±≤ζΈο÷– «Ζώ¥φ‘ΎΕΓΟ―![]() «κΤάΦέΗΟΆ§―ß…ηΦΤΒΡΦχΕ®ΖΫΑΗ «ΖώΚœάμΘΩάμ”… «______ΓΘ
«κΤάΦέΗΟΆ§―ß…ηΦΤΒΡΦχΕ®ΖΫΑΗ «ΖώΚœάμΘΩάμ”… «______ΓΘ
(5)ΈΣΝΥΫχ“Μ≤ΫΧα¥Ω1“ΜδεΕΓΆιΘ§ΗΟ–ΓΉιΆ§―ß≤ιΒΟœύΙΊ”–ΜζΈοΒΡ”–ΙΊ ΐΨί»γ±μΘΚ
Έο÷ | »έΒψ | Ζ–Βψ |
1“ΜΕΓ¥Φ |
|
|
1“ΜδεΕΓΆι |
|
|
ΕΓΟ― |
|
|
1“ΜΕΓœ© |
|
|
‘ρ”ΟBΉΑ÷ΟΆξ≥…¥ΥΧα¥Ω Β―ι ±ΘΜΘ§ Β―ι÷–“Σ―ΗΥΌ…ΐΗΏΈ¬Ε»÷Ν______ ’Φ·ΥυΒΟΝσΖ÷ΓΘ
(6)»τ Β―ι÷–Υυ»Γ1“ΜΕΓ¥ΦΓΔNaBrΖ÷±πΈΣ![]() ΓΔ
ΓΔ![]() Θ§≈®ΝρΥα
Θ§≈®ΝρΥα![]() Θ§’τ≥ωΒΡ¥÷≤ζΈοΨ≠œ¥Β”Θ§Η…‘οΚσ‘Ό¥Έ’τΝσΒΟΒΫ
Θ§’τ≥ωΒΡ¥÷≤ζΈοΨ≠œ¥Β”Θ§Η…‘οΚσ‘Ό¥Έ’τΝσΒΟΒΫ![]() “ΜδεΕΓΆιΘ§‘ρ1“ΜδεΕΓΆιΒΡ≤ζ¬ «______
“ΜδεΕΓΆιΘ§‘ρ1“ΜδεΕΓΆιΒΡ≤ζ¬ «______![]() ±ΘΝτ2ΈΜ”––ß ΐΉ÷
±ΘΝτ2ΈΜ”––ß ΐΉ÷![]() ΓΘ
ΓΘ



