��Ŀ����
ʵ����ͨ����Ũ������Ҵ���Ӧ����ȡ��ϩ�������¶ȹ��߶����������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ��������Իش��������⣮
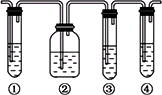
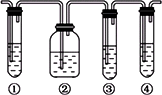
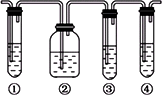
(1)��ͼ�Т١��ڡ��ۡ���װ�ÿ�ʢ�ŵ��Լ��ֱ��ǣ�
��________����________����________����________(�������й��Լ�������������)��
A��Ʒ����Һ��B��NaOH��Һ��C��ŨH2SO4��D������KMnO4��Һ
(2)��˵����������������ڵ�������________________��
(3)ʹ��װ�âں͢۵�Ŀ����________________��
(4)˵��������ϩ��������________________��
������
|
�����𰸣�(1)A��B��A��D ����(2)װ�â���Ʒ����Һ��ɫ ����(3)��ȥSO2���壬���������ϩ������ʵ�飻����SO2�Ƿ��� ����(4)װ�â��е�Ʒ����Һ����ɫ��װ�â��е�����KMnO4��Һ��ɫ ����˼·�㲦��
����������𣺸�ʵ���Ŀ���ǣ�ȷ�ϻ������������ϩ��SO2��������ϩ��SO2����ʹ����KMnO4��Һ��ɫ����������ϩ����NaOH��Һ��Ӧ����SO2����NaOH��Һ��Ӧ�����ʳ�ȥSO2����Ʒ����Һ�ɼ���SO2�Ĵ��ں��Ƿ��������ȥSO2������������KMnO4��Һ�Ƿ���ɫ��ȷ����ϩ�Ĵ��ڣ� |

 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�Na2SO3�dz��õĿ�������
��1��ʵ����ͨ����Ũ���ᣨ1��1����Na2SO3���Ʊ�SO2���壬
��Ӧ����ʽΪ�� ���Ʊ���SO2������ͨ������ˮ���������и�����ܸ���SO2������ǣ� ��
A.Ũ���� B.��ʯ�� C.��ˮCaCl2
��2�� ����SO2����ͨ��NaOH��Һ�пɵ�NaOH��Na2SO3�Ļ����Һ����û����Һ�м���������ˮ������Һ��Ϊ��ɫ��������Һ��Br2��Na2SO3����������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ______________��
��3����Ӧ�����Һ����SO32����SO42����Br����OH���������ӣ�����д��������SO32����SO42����Br����ʵ�鱨�棻
��ѡ�Լ���2 mol��L��1HCl��1 mol��L��1H2SO4��1mol��L��1HNO3��1 mol��L��1BaCl2��
1 mol��L��1Ba(NO3)2��0.1 mol��L��1AgNO3��CCl4���������Ʊ�����ˮ�����Ʊ�����ˮ��
|
��� |
ʵ����� |
Ԥ������ͽ��� |
|
����� |
ȡ��������Һ���Թ�A�У��μ�2 mol��L��1HCl����Һ�����ԣ����뼸��________(���Լ�)���� |
________��֤������Һ�к�SO32- |
|
����� |
��ȡ��������Һ���Թ�B�У����� ���ٵμ����� 1 mol��L��1 BaCl2��Һ |
|
|
����� |
��ȡ��������Һ���Թ�C�У� �������ú�۲���ɫ |
��Һ�ֲ㣬�ϲ�Һ��ʳȺ�ɫ��֤������Һ�к�Br- |