��Ŀ����
����Ŀ�����仯����������������Ӧ�ù㷺���ش��������⣺
(1)��立���һ�ֻ�ѧ����,����ʽ�� NH4Fe(SO4)2��12H2O,������ˮ��,��Һ�е�����Ũ�ȴ�С��ϵΪ__________________��
(2)��֪ij��Һ�к��� CO32����SO42��������,ȡһ�����ĸ���Һ,�����еμ�BaCl2��Һ���� CO32����ʼ�� ��ʱ����Һ��c(CO32-)/c(SO42-)Ϊ_______________��(��֪ Ksp(BaSO4 )��1.0��10��10 ��Ksp(BaCO3)��2.5��10��9 )
(3)��֪��S2Cl2(l)��Cl2(g)��2SCl2(l) ��H����50.2kJ��mol��1 ������ 1molCl��Cl����1molS��S���ֱ���Ҫ���� 243kJ��268kJ ������������� 1mol S��Cl����Ҫ���յ�����Ϊ____kJ��
(4)�� NaOH ��Һ���������е� SO2,�����õ� Na2SO3 ��Һ���е��,�����Ʊ�H2SO4����ԭ������ͼ��ʾ(�缫����Ϊʯī)��
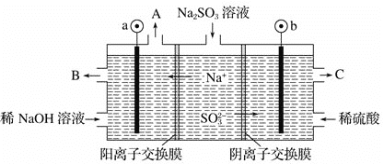
�����ĵ缫��ӦʽΪ______________________�����п�ѭ��ʹ�õ�������________��
���𰸡�c(SO42��)>c(NH4��)>c(Fe3��)>c(H��)>c(OH��) 25 280.6 SO32����2e����H2O=SO42����2H�� NaOH
��������
��1���������NH4Fe(SO4)2��12H2O����ˮ�γɵ���Һ�У�Fe3����NH4��ˮ����Һ�����ԣ�Fe3����ˮ��̶ȴ���NH4����ˮ��̶�Խ������Ũ��ԽС���ݴ˽��
��2������c(CO32-)/c(SO42-)= Ksp(BaCO3)/ Ksp(BaSO4 )������
��3����Ӧ���ʱ���ڷ�Ӧ��ļ��ܼ�ȥ������ļ��ܣ������1mol S��Cl����Ҫ���յ�����Ϊx���з��������
��4��ͼ����������b���ƶ�����bΪ����������b����SO32��������ʧ��������SO42�����ݴ˽����
��1���������NH4Fe(SO4)2��12H2O����ˮ�γɵ���Һ�У�Fe3����NH4��ˮ����Һ�����ԣ�Fe3����ˮ��̶ȴ���NH4����ˮ��̶�Խ������Ũ��ԽС������c(NH4��)>c(Fe3��)����������Ӳ�ˮ�⣬Ũ�����������Һ������Ũ�ȹ�ϵc(SO42��)>c(NH4��)>c(Fe3��)>c(H��)>c(OH��)��
��ˣ�������ȷ������c(SO42��)>c(NH4��)>c(Fe3��)>c(H��)>c(OH��)��
��2������c(CO32-)/c(SO42-)= Ksp(BaCO3)/ Ksp(BaSO4 )��֪��c(CO32-)/c(SO42-)=![]() 25��
25��
��ˣ�������ȷ������25��
��3����Ӧ���ʱ���ڷ�Ӧ��ļ��ܼ�ȥ������ļ��ܣ������1mol S��Cl����Ҫ���յ�����Ϊx����2x+268 kJ+243 kJ-4x=-50.2 kJ�����x=280.6 kJ��
��ˣ�������ȷ������280.6��
��4��ͼ����������b���ƶ�����bΪ����������b����SO32��������ʧ��������SO42������缫����ʽΪ��SO32����2e����H2O=SO42����2H����aΪ�������������ŵ�����Ϊ����������������B��������������Ũ�Ƚϴ������������Һ�����������ն������ʿ�ѭ��ʹ�õ�������NaOH��
��ˣ�������ȷ������SO32����2e����H2O=SO42����2H�� ��NaOH��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�