��Ŀ����
20����һ���ۡ�����ͭ����������ɵĻ�������Ͷ�뵽110mL 4mol•L-1�������г�ַ�Ӧ�ų�����896mL����״���²ⶨ�����������ù���������أ�������Ϊ1.28g����Һ����������ȷ��������Cu2+������Һϡ�͵�200mL������ȡ��50mL������1mol•L-1��NaOH��Һ����������20mL ��������Һ�м�NaOH��Һ����ֳ����������������۵��������������� ��Ӧ����Һ�л��н϶�H+����ʣ�࣬����Һ��û��Cu2+����֪������û��Fe��ֻ��Cu����Cu���Բ���˵����Һ��û��Fe3+����Һ��������ΪH+��Fe2+���μӷ�Ӧ��HCl�е�HԪ��ת����������ˮ�У�����Hԭ���غ㣬����������Oԭ�����ʵ���������CuԪ���غ����n��CuO�����ٸ���Oԭ���غ����n��Fe2O3��������$\frac{m�����ۣ�}{������������}��100%$����������۵�����������
��� �⣺��Ӧ����Һ�л��н϶�H+����ʣ�࣬����Һ��û��Cu2+����֪������û��Fe��ֻ��Cu����Cu���Բ���˵����Һ��û��Fe3+����Һ��������ΪH+��Fe2+��
��Ӧ��ʣ��n��H+��=1mol/L��0.02L��$\frac{200}{50}$=0.08mol��ԭ��Һ��n��HCl��=4mol/L��0.11L=0.44mol��
�μӷ�Ӧ��HCl�е�HԪ��ת����������ˮ�У�����Hԭ���غ㣬2n��H2O��+2n��H2��=n����HCl��-n����H+������2n��H2O��+2��$\frac{0.896L}{22.4L/mol}$=0.44mol-0.08mol��n��H2O��=0.14mol���ʻ������n��O��=n��H2O��=0.14mol��
��ͭԪ���غ㣬��n��CuO��=n��Cu��=$\frac{1.28g}{64g/mol}$=0.02mol��
��Oԭ���غ㣺3n��Fe2O3��+n��CuO��=0.14mol����n��Fe2O3��=0.04mol��
����Clԭ���غ㣺n����HCl��=nʣ����HCl��+2n��FeCl2������0.11L��4mol/L=0.08mol+2n��FeCl2�������n��FeCl2��=0.18mol������n��Fe��+2n��Fe2O3��=0.18mol����n��Fe��=0.1 mol�����Ի���������۵���������Ϊ��$\frac{0.1��56}{0.02��80+0.04��160+0.1��56}��100%$=41.2%���𣺺��������۵���������Ϊ��41.2%��
���� ���⿼��������㣬ע�����Һ���ڵ������ж����ʷ�Ӧ�ij̶ȣ�ע�������غ�ķ������㣬��Ŀ�Ѷ��еȣ�

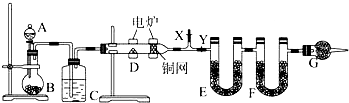
| A�� |  �Ʊ�Fe��OH��2���۲���ɫ | B�� |  �������еĵ�;ƾ� | ||
| C�� |  ��ȥCl2�е�HCl | D�� |  ���� |
| A�� | ���������۵�ܸߣ����Բ���������ұ���� | |
| B�� | ����������һ�ֽ�״�������нϴ��������������ԣ���������ˮ�� | |
| C�� | ʵ���ҿ����������������Ȼ������Ʊ��������� | |
| D�� | ���������ȿ���ǿ�ᷴӦ�ֿ���ǿ�Ӧ���������������� |