��Ŀ����
��1��һ��������CH3COOH��Һ�еĵ���ƽ��Ϊ��CH3COOH?CH3COO-+H+��H��0
���з����У�����ʹ0.10mol?L-1CH3COOH��Һ��CH3COOH����̶��������
a����������0.10mol?L-1��ϡ���� b������CH3COOH��Һ
c����ˮϡ����0.010mol?L-1 d����������������
e�����������Ȼ��ƹ��� f����������0.10mol?L-1��NaOH��Һ
��2��25��ʱ���õ���ƽ���ƽ�ⳣ��Ϊ1.75��10-5�����У���0.10mol?L-1CH3COOH��Һ ��0.0010mol?L-1 CH3COOH��Һ
��c��H+��֮��Ϊ�٣���=
��3��25��ʱ����0.10mol?L-1CH3COOH��Һ�м���һ��������CH3COONa��������Һ������䣩������Һ��c��CH3COO-��Ϊ1.0mol?L-1������Һ��c��H+��=
���з����У�����ʹ0.10mol?L-1CH3COOH��Һ��CH3COOH����̶��������
bcf
bcf
��a����������0.10mol?L-1��ϡ���� b������CH3COOH��Һ
c����ˮϡ����0.010mol?L-1 d����������������
e�����������Ȼ��ƹ��� f����������0.10mol?L-1��NaOH��Һ
��2��25��ʱ���õ���ƽ���ƽ�ⳣ��Ϊ1.75��10-5�����У���0.10mol?L-1CH3COOH��Һ ��0.0010mol?L-1 CH3COOH��Һ
��c��H+��֮��Ϊ�٣���=
10��1
10��1
������ʾ�����м��㣬ƽ��ʱ��c��CH3COOH��������ʼŨ�ȴ��棬ˮ�������c��H+����c��OH-�����Բ��ƣ���ͬ����3��25��ʱ����0.10mol?L-1CH3COOH��Һ�м���һ��������CH3COONa��������Һ������䣩������Һ��c��CH3COO-��Ϊ1.0mol?L-1������Һ��c��H+��=
1.75��10-6
1.75��10-6
mol?L-1������������С�������λ��Ч���֣�����Һ�и�������Ũ�ȹ�ϵ��c��CH3COO-����c��Na+����c��H+����c��OH-��
c��CH3COO-����c��Na+����c��H+����c��OH-��
����������1������ĵ��������ȷ�Ӧ����ˮϡ�͡��������ȶ��ܴٽ�����ĵ��룻
��2��C��H+��=
���ݴ˼���������Ũ��֮�ȣ�
��3��C��H+��=
����Һ�����ԣ���Һ��������Ũ�ȴ�������������Ũ�ȣ����ݵ���غ�ȷ�����������Ũ�Ⱥ�������Ũ�ȴ�С��
��2��C��H+��=
| kaC(CH3COOH) |
��3��C��H+��=
| Ka��C(CH3COOH) |
| C(CH3COO-) |
����⣺��1������ĵ��������ȷ�Ӧ����ˮϡ�͡��������ȶ��ܴٽ�����ĵ��룻
a����������0.10mol?L-1��ϡ���ᣬ��Һ��������Ũ���������ƴ���ĵ��룬�����ĵ���̶Ƚ��ͣ��ʴ���
b������ĵ��������ȷ�Ӧ������CH3COOH��Һ���ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
c����ˮϡ����0.010mol?L-1���ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
d���������������ᣬ����ĵ���ƽ��������Ӧ�����ƶ���������ĵ���̶Ƚ��ͣ��ʴ���
e�����������Ȼ��ƹ��壬��Ӱ��ƽ����ƶ����ı����ĵ��룬�ʴ���
f����������0.10mol?L-1��NaOH��Һ�����������Ӻ������ӷ�Ӧ����ˮ��������Ũ�Ƚ��ͣ��ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
��ѡbcf��
��2��0.10mol?L-1CH3COOH��Һ��C��H+��=
mol/L=
mol/L=
��10-3mol/L��0.0010mol?L-1 CH3COOH��Һ��C��H+��=
mol/L=
mol/L=
��10-4mol/L����c��H+��֮��Ϊ�٣���=
��10-3mol/L��
��10-4mol/L=10��1��
�ʴ�Ϊ��10��1��
��3����������Ũ��Ϊx��C��H+��=
=
mol/L=x������ĵ���̶Ⱥ�С������0.1-xԼ����0.1������x=1.75��10-6 mol/L��
��Һ�����ԣ���Һ��������Ũ�ȴ�������������Ũ�ȣ����ݵ���غ�֪c��CH3COO-����c��Na+����������Һ�Ǵ����ƺʹ�������Һ��������Ũ�Ƚ�С������c��Na+����c��H+�������������Ũ�ȴ�С˳����c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��1.75��10-6��c��CH3COO-����c��Na+����c��H+����c��OH-����
a����������0.10mol?L-1��ϡ���ᣬ��Һ��������Ũ���������ƴ���ĵ��룬�����ĵ���̶Ƚ��ͣ��ʴ���
b������ĵ��������ȷ�Ӧ������CH3COOH��Һ���ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
c����ˮϡ����0.010mol?L-1���ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
d���������������ᣬ����ĵ���ƽ��������Ӧ�����ƶ���������ĵ���̶Ƚ��ͣ��ʴ���
e�����������Ȼ��ƹ��壬��Ӱ��ƽ����ƶ����ı����ĵ��룬�ʴ���
f����������0.10mol?L-1��NaOH��Һ�����������Ӻ������ӷ�Ӧ����ˮ��������Ũ�Ƚ��ͣ��ٽ�����ĵ��룬�����ĵ���̶�������ȷ��
��ѡbcf��
��2��0.10mol?L-1CH3COOH��Һ��C��H+��=
| kaC(CH3COOH) |
| 1.75��10-5��0.10 |
| 1.75 |
| kaC(CH3COOH) |
| 1.75��10-5��0.0010 |
| 1.75 |
| 1.75 |
| 1.75 |
�ʴ�Ϊ��10��1��
��3����������Ũ��Ϊx��C��H+��=
| Ka��C(CH3COOH) |
| C(CH3COO-) |
| 1.75��10-5��(0.10-x) |
| 1.0 |
��Һ�����ԣ���Һ��������Ũ�ȴ�������������Ũ�ȣ����ݵ���غ�֪c��CH3COO-����c��Na+����������Һ�Ǵ����ƺʹ�������Һ��������Ũ�Ƚ�С������c��Na+����c��H+�������������Ũ�ȴ�С˳����c��CH3COO-����c��Na+����c��H+����c��OH-����
�ʴ�Ϊ��1.75��10-6��c��CH3COO-����c��Na+����c��H+����c��OH-����
���������⿼����������ʵĵ��롢������ʵ��볣�����йؼ��㡢����Ũ�ȴ�С�ıȽϵ�֪ʶ�㣬ע�⣨1���м������������ᣬ����ĵ���ƽ��������Ӧ�����ƶ���������ĵ���̶Ƚ��ͣ�Ϊ�״��㣮

��ϰ��ϵ�д�
 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ

 ����дһ�֣�
����дһ�֣�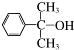 Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��
Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��