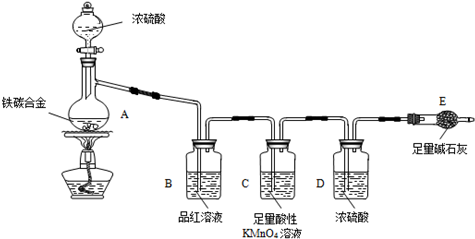
��Ŀ����
ijʵ����ȤС��Ϊ�˲ⶨ��п��Ƥ��п�����������أ�Zn�����������ʵ�鷽����
�������ס��ⶨ������6mol/L������Һ��Ӧǰ�����������仯���������£�

��1������II�жƲ㷴Ӧ��ı�־��______������IV��������______��
��2������III�У�������Ƭ��ϴ���ķ�����______��
��3����Ҫ��һ������п�Ʋ��ȣ�����֪�����������ǣ���п��Ƥ�ı����S��______��
�������ҡ��ⶨ������6mol/L���ռ���Һ��Ӧ���ɵ����������װ����ͼ��

[��֪��п������ǿ����Һ��Zn+2NaOH+2H2O��Na2Zn��OH��4+H2��]
��4������250mL 6mol/L��NaOH��Һ����Ҫ��ȡ48%��ŨNaOH��Һ���ܶ�Ϊ1.506g/cm3��______mL���ù��Ϊ______mL��Ͳ��ȡ��
��5��Ϊ��ȷ�ⶨH2������ڷ�Ӧ��������ȴ������Ͳ�ڴ�Һƿ����齺�ܴ����г�����ֱ��______Ϊֹ����ʡ�Դ˲��裬��õ�������ݽ�______���ƫ����ƫС�����䡱����
��6����֪��п��Ƥ������Ϊm g�������ռ���Һ�����ΪV1mL������������ݾ�����������״������ͬ��������Һ����ƿ��ˮ�����ΪV2mL����Ͳ�����������ΪV3mL��ѡ��������ݣ�����أ�Zn��=______��
�⣺��1���ⶨ������6mol/L������Һ��Ӧǰ�����������仯����Ӧ��ȫ�Dz��������������õ��������ʯ�������þƾ��ƺ�ɣ�
�ʴ�Ϊ����������������ͻȻ��������ɣ�
��2������III�У�������Ƭ��ϴ���ķ����Ǽ���ϴ��Һ���Ƿ��������ӣ�ȡ���һ��ϴ��Һ��������������Һ���ް�ɫ��������������Ƭ��ϴ����
�ʴ�Ϊ��ȡ���һ��ϴ��Һ��������������Һ���ް�ɫ��������������Ƭ��ϴ����
��3������п�Ʋ��ȣ�ʵ��ⶨп������������֪�����������ǣ���п��Ƥ�ı����S������п���ܶȣ��ʴ�Ϊ������п���ܶȣ�
��4������250mL 6mol/L��NaOH��Һ����Ҫ��ȡ48%��ŨNaOH��Һ���ܶ�Ϊ1.506g/cm3����Һ���Ϊx���õ�0.250ml��6mol/L=��x��1.506g/mol��48%���� ���õ�x=8ml��ѡ����Ͳ��100ml��
���õ�x=8ml��ѡ����Ͳ��100ml��
�ʴ�Ϊ��83��100��
��5������Ͳ�ڴ�Һƿ����齺�ܴ����г�����֤���ɵ�����ȫ�����봢��ƿ�����Դ˲���ⶨ���ƫС���ʴ�Ϊ����Һƿ�е�������Һ����ƽ��ƫС��
��6��Zn+2NaOH+2H2O��Na2Zn��OH��4+H2������Ӧ��п���ʵ�����������ͬ����֪��п��Ƥ������Ϊm g�������ռ���Һ�����ΪV1mL������������ݾ�����������״������ͬ��������Һ����ƿ��ˮ�����ΪV2mL����Ͳ�����������ΪV3mL���������ʵ���=�� ��10-3+
��10-3+ ��10-3��mol��
��10-3��mol��
���������= ��100%=
��100%= ��100%
��100%
�ʴ�Ϊ�� ��100%��
��100%��
��������1�������ܽ�п��Ӧ��ȫ��������ų����������̷������ⶨ������6mol/L������Һ��Ӧǰ�����������仯���������ǶԹ����ɣ�
��2������ϴ��Һ�������ӵĴ�������������Ƿ�ϴ����
��3������п�Ʋ��ȣ���Ҫ��������п��������п��Ƥ�ı����S���ܶȣ�
��4��������Һ���������ʵ�����ͬ���㣬���ݼ���õ���Һ�������Ͳ�Ĺ��ѡ��
��5������Ͳ�ڴ�Һƿ����齺�ܴ����г�����֤���ɵ�����ȫ�����봢��ƿ�����Դ˲���ⶨ���ƫС��
��6���������ɵ��������ʵ�����Һ����ƿ��ˮ�����ΪV2mL����Ͳ�����������ΪV3mL����ϻ�ѧ����ʽ����п�����ʵ�����Ϻ�п������������
���������⿼�����������ʵ�̽��ʵ�鷽���Ͳ����������Һ���Ƶ�����Ӧ�ã���ѧ����ʽ�ļ��㣬��Ŀ�Ѷ��еȣ�
�ʴ�Ϊ����������������ͻȻ��������ɣ�
��2������III�У�������Ƭ��ϴ���ķ����Ǽ���ϴ��Һ���Ƿ��������ӣ�ȡ���һ��ϴ��Һ��������������Һ���ް�ɫ��������������Ƭ��ϴ����
�ʴ�Ϊ��ȡ���һ��ϴ��Һ��������������Һ���ް�ɫ��������������Ƭ��ϴ����
��3������п�Ʋ��ȣ�ʵ��ⶨп������������֪�����������ǣ���п��Ƥ�ı����S������п���ܶȣ��ʴ�Ϊ������п���ܶȣ�
��4������250mL 6mol/L��NaOH��Һ����Ҫ��ȡ48%��ŨNaOH��Һ���ܶ�Ϊ1.506g/cm3����Һ���Ϊx���õ�0.250ml��6mol/L=��x��1.506g/mol��48%����
 ���õ�x=8ml��ѡ����Ͳ��100ml��
���õ�x=8ml��ѡ����Ͳ��100ml���ʴ�Ϊ��83��100��
��5������Ͳ�ڴ�Һƿ����齺�ܴ����г�����֤���ɵ�����ȫ�����봢��ƿ�����Դ˲���ⶨ���ƫС���ʴ�Ϊ����Һƿ�е�������Һ����ƽ��ƫС��
��6��Zn+2NaOH+2H2O��Na2Zn��OH��4+H2������Ӧ��п���ʵ�����������ͬ����֪��п��Ƥ������Ϊm g�������ռ���Һ�����ΪV1mL������������ݾ�����������״������ͬ��������Һ����ƿ��ˮ�����ΪV2mL����Ͳ�����������ΪV3mL���������ʵ���=��
 ��10-3+
��10-3+ ��10-3��mol��
��10-3��mol�����������=
 ��100%=
��100%= ��100%
��100%�ʴ�Ϊ��
 ��100%��
��100%����������1�������ܽ�п��Ӧ��ȫ��������ų����������̷������ⶨ������6mol/L������Һ��Ӧǰ�����������仯���������ǶԹ����ɣ�
��2������ϴ��Һ�������ӵĴ�������������Ƿ�ϴ����
��3������п�Ʋ��ȣ���Ҫ��������п��������п��Ƥ�ı����S���ܶȣ�
��4��������Һ���������ʵ�����ͬ���㣬���ݼ���õ���Һ�������Ͳ�Ĺ��ѡ��
��5������Ͳ�ڴ�Һƿ����齺�ܴ����г�����֤���ɵ�����ȫ�����봢��ƿ�����Դ˲���ⶨ���ƫС��
��6���������ɵ��������ʵ�����Һ����ƿ��ˮ�����ΪV2mL����Ͳ�����������ΪV3mL����ϻ�ѧ����ʽ����п�����ʵ�����Ϻ�п������������
���������⿼�����������ʵ�̽��ʵ�鷽���Ͳ����������Һ���Ƶ�����Ӧ�ã���ѧ����ʽ�ļ��㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ



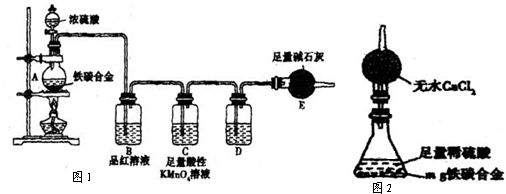
 ��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ�������
��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ�������