��Ŀ����
�о����ϣ���ʦ��ͬѧ�Ǹ���ѡ����м��㣬������ȷ����
- A.��ͬѧ��0.2molMgO�������㣬������Ϊ0.2g
- B.��ͬѧ��9.03��1023��O2�������㣬O2���ʵ���Ϊ1.5mol
- C.��ͬѧ����״���£�5.6Lˮ�������������Ϊ0.25mol
- D.��ͬѧ����30mL0.5mol?L-1NaOH��Һ��ˮϡ�͵�500mL�������㣬ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ0.04mol?L-1
B
������A������m=n��M����������þ��������
B������n=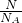 ������O2���ʵ�����
������O2���ʵ�����
C����״���£�ˮΪҺ�壻
D������ϡ��ǰ�����ʵ����ʵ������������㣮
���A��MgO������Ϊ0.2mol��40g/mol=8g����A����
B��O2���ʵ���Ϊ =1.5mol����B��ȷ��
=1.5mol����B��ȷ��
C����״���£�ˮΪҺ�壬��������22.4L/mol�����㣬��C����
D����ϡ��ǰ�����ʵ����ʵ������䣬ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ =0.03mol?L-1����D����
=0.03mol?L-1����D����
��ѡB��
���������⿼���й����ʵ����ļ��㣬��ȷ�����������������Ũ�������ʵ����Ĺ�ϵ���ɽ��
������A������m=n��M����������þ��������
B������n=
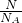 ������O2���ʵ�����
������O2���ʵ�����C����״���£�ˮΪҺ�壻
D������ϡ��ǰ�����ʵ����ʵ������������㣮
���A��MgO������Ϊ0.2mol��40g/mol=8g����A����
B��O2���ʵ���Ϊ
 =1.5mol����B��ȷ��
=1.5mol����B��ȷ��C����״���£�ˮΪҺ�壬��������22.4L/mol�����㣬��C����
D����ϡ��ǰ�����ʵ����ʵ������䣬ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ
 =0.03mol?L-1����D����
=0.03mol?L-1����D������ѡB��
���������⿼���й����ʵ����ļ��㣬��ȷ�����������������Ũ�������ʵ����Ĺ�ϵ���ɽ��

��ϰ��ϵ�д�
�����Ŀ
|
�о����ϣ���ʦ��ͬѧ�Ǹ���ѡ����м��㣬������ȷ���� | |
| [����] | |
A�� |
��ͬѧ��0.2 mol��MgO�������㣬������Ϊ0.2 g |
B�� |
��ͬѧ��9.03��1023��O2�������㣬O2���ʵ���Ϊ1.5 mol |
C�� |
��ͬѧ����״���£�5.6 Lˮ�������������Ϊ0.25 mol |
D�� |
��ͬѧ����30 mL��0.5 mol��L��1��NaOH��Һ��ˮϡ�͵�500 mL�������㣬ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ0.04 mol��L��1 |
�о����ϣ���ʦ��ͬѧ�Ǹ���ѡ����м��㣬������ȷ����
A����ͬѧ��0 .2 mol MgO�������㣬������Ϊ0.2 g .2 mol MgO�������㣬������Ϊ0.2 g |
B����ͬѧ��9.03��1023��O2�������㣬O2���ʵ��� Ϊ1.5 mol Ϊ1.5 mol |
C����ͬѧ����״���£�5.6  L ˮ�������������Ϊ 0.25 mol L ˮ�������������Ϊ 0.25 mol |
| D����ͬѧ����30 mL0.5mol��L-1 NaOH��Һ��ˮϡ�͵�500mL�������㣬ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ0.04mol��L-1 |