��Ŀ����
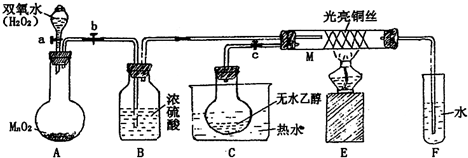
�йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ�ã��г�װ��������ʡ�ԣ�����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a��b��c��ͨ�����ƻ���a��b�����н��ࣨ��Ъ�ԣ�ͨ�����壬������M���۲쵽���Ե�ʵ������
��1��A�з�����Ӧ�Ļ�ѧ����ʽ��_______________________________ ��
B�����ã�________________________��
��2��M�������ķ�Ӧ�Ļ�ѧ����ʽΪ��______________________________________
��3����ʵ������д���______________ ����μӡ����μӡ����˻�ѧ��Ӧ
��4��ʵ���Ҵ�����������Լ��� ����д����Ӧ�Ļ�ѧ����ʽ
��
��5�����Թ�F���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л���
�� ��Ҫ��ȥ�����ʣ������ڻ��Һ�м��� ����д��ĸ����
| A���Ȼ�����Һ | B���� | C��̼��������Һ | D�����Ȼ�̼ |
��1��2H2O2  2H2O+O2����2�֣� B�����ø���O2��2�֣���
2H2O+O2����2�֣� B�����ø���O2��2�֣���
��2��2CH3CH2OH+O2  2CH3CHO+2H2O (2��,��д�ֲ���Ӧ)
2CH3CHO+2H2O (2��,��д�ֲ���Ӧ)
��3���μӣ�1�֣���
��4������������ͭ����Һ��������Һ������1�֣�
CH3CHO+2Ag��NH3��2OH CH3COONH4+2Ag��+3NH3+H2O
CH3COONH4+2Ag��+3NH3+H2O
��CH3CHO+2Cu��OH��2 CH3COOH+Cu2O��+2H2O��2�֣�
CH3COOH+Cu2O��+2H2O��2�֣�
��5��CH3COOH��1�֣���C��1�֣�
����



 ɫ��___________________________________________��
ɫ��___________________________________________��
