��Ŀ����
 ��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ���������������ش��������⣺
��1913�깤ҵ�ϳɰ�Ͷ���������ϳɰ���ҵ���Ϸ�չ�����ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ�Ͽɽ���������������ش��������⣺��1����֪��N2��g��+O2��g��=2NO��g����H=+180.5kJ/molN2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
д������������������һ�����������ˮ�������Ȼ�ѧ����ʽ��
4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol
4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol
����2��ij�о�С����673K��30MPa�����£������ΪVL���ܱ������н��з�Ӧ��
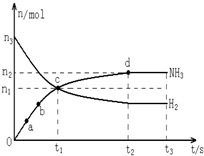
N2��g��+3H2��g��?2NH3��g������n��H2����n��NH3����ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
����������ȷ����
AC
AC
��������ĸ��A����a������Ӧ���ʱȵ�b�Ĵ�
B����c����Ӧ�ﵽ��ѧƽ��״̬
C����t3ʱ��673K����773K����n��H2��������
D��t2��t3ʱ�̣�n��N2�������
��3����һ���¶Ⱥʹ����£���6.4mol H2��2.4molN2�����һ���ݻ�Ϊ4L���ܱ������з�����Ӧ����3minĩʱ��Ӧǡ�ô�ƽ�⣬��ʱ������1.6mol NH3�����㣺��д��������̣���3min����H2��ʾ�Ļ�ѧ��Ӧ���ʣ��ڸ������µ�ƽ�ⳣ����
��������1������֪�Ȼ�ѧ����ʽ���ݸ�˹���ɹ���Ŀ���Ȼ�ѧ����ʽ��
��2��A�����������ʵ���ԽС����Ӧ������ʵ���Խ��Ӧ��Ũ��Խ������Ӧ����Խ�죮
B��c���Ժ��������������ʵ��������仯��
C�������¶�ƽ�������ȷ����ƶ���
D��t2��t3ʱ�̣����淴Ӧ����ƽ��״̬��
��3����������ʽ����������ʵ�Ũ�ȱ仯��ƽ��ʱ�����ʵ�Ũ�ȣ�
�ٸ���v=
����v��H2����
��ƽ�ⳣ��k=
����ƽ��Ũ�ȴ�����㣮
��2��A�����������ʵ���ԽС����Ӧ������ʵ���Խ��Ӧ��Ũ��Խ������Ӧ����Խ�죮
B��c���Ժ��������������ʵ��������仯��
C�������¶�ƽ�������ȷ����ƶ���
D��t2��t3ʱ�̣����淴Ӧ����ƽ��״̬��
��3����������ʽ����������ʵ�Ũ�ȱ仯��ƽ��ʱ�����ʵ�Ũ�ȣ�
�ٸ���v=
| ��c |
| ��t |
��ƽ�ⳣ��k=
| c2(NH3) |
| c(N2)?c3(H2) |
����⣺��1����֪����N2��g��+O2��g��=2NO��g����H=+180.5kJ/mol
��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
�ɸ�˹���ɿ�֪���١�2-�ڡ�2+�ۡ�3�ã�4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol
�ʴ�Ϊ��4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol��
��2��A����ͼ��֪�����ڵ�a���ڵ�bʱ�����ʵ���С����a��ʱ��Ӧ���Ũ�ȴ�Ӧ��Ũ��Խ������Ӧ����Խ�죬�ʵ�a������Ӧ���ʱȵ�b�Ĵ�A��ȷ��
B����ͼ��֪c��������ʵ����������������ʵ�����С��c��δ����ƽ��״̬��ƽ��������Ӧ���У���B����
C���ϳɰ�����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�������ȷ����ƶ��������淴Ӧ�ƶ�����n��H2������C��ȷ��
D��t2��t3ʱ�̣����淴Ӧ������ͬƽ��״̬��n��N2����ȣ���D����
��ѡ��AC��
��3�����ڿ��淴Ӧ��N2��g��+3H2��g�� 2NH3��g��
2NH3��g��
��ʼ���ʵ���Ũ�ȣ�mol/L�� 0.6 1.6 0
ת�����ʵ���Ũ�ȣ�mol/L�� 0.2 0.6 0.4
ƽ�����ʵ���Ũ�ȣ�mol/L�� 0.4 1 0.4
��3min����H2��ʾ�Ļ�ѧ��Ӧ����v��H2��=
=0.2mol/��L?min����
��3min����H2��ʾ�Ļ�ѧ��Ӧ����Ϊ0.2mol/��L?min����
��ƽ�ⳣ��k=
=
=0.4L2/mol2��
�𣺸������µ�ƽ�ⳣ��Ϊ0.4L2/mol2��
��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ/mol
��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ/mol
�ɸ�˹���ɿ�֪���١�2-�ڡ�2+�ۡ�3�ã�4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol
�ʴ�Ϊ��4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=-905.0kJ/mol��
��2��A����ͼ��֪�����ڵ�a���ڵ�bʱ�����ʵ���С����a��ʱ��Ӧ���Ũ�ȴ�Ӧ��Ũ��Խ������Ӧ����Խ�죬�ʵ�a������Ӧ���ʱȵ�b�Ĵ�A��ȷ��
B����ͼ��֪c��������ʵ����������������ʵ�����С��c��δ����ƽ��״̬��ƽ��������Ӧ���У���B����
C���ϳɰ�����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�������ȷ����ƶ��������淴Ӧ�ƶ�����n��H2������C��ȷ��
D��t2��t3ʱ�̣����淴Ӧ������ͬƽ��״̬��n��N2����ȣ���D����
��ѡ��AC��
��3�����ڿ��淴Ӧ��N2��g��+3H2��g��
 2NH3��g��
2NH3��g����ʼ���ʵ���Ũ�ȣ�mol/L�� 0.6 1.6 0
ת�����ʵ���Ũ�ȣ�mol/L�� 0.2 0.6 0.4
ƽ�����ʵ���Ũ�ȣ�mol/L�� 0.4 1 0.4
��3min����H2��ʾ�Ļ�ѧ��Ӧ����v��H2��=
| 0.6mol/L |
| 3min |
��3min����H2��ʾ�Ļ�ѧ��Ӧ����Ϊ0.2mol/��L?min����
��ƽ�ⳣ��k=
| c2(NH3) |
| c(N2)?c3(H2) |
| (0.4mol/L)2 |
| 0.4mol/L?(1mol/L)3 |
�𣺸������µ�ƽ�ⳣ��Ϊ0.4L2/mol2��
�����������Ȼ�ѧ����ʽ��д��Ӱ��ƽ���ƶ������ء�ƽ��ͼ��ѧƽ�����ȣ��Ѷ��еȣ�ע����������ʽ���ⷨ���˹���ɣ�

��ϰ��ϵ�д�
�����Ŀ
