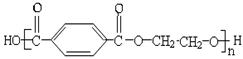
��Ŀ����
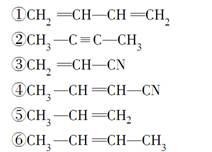
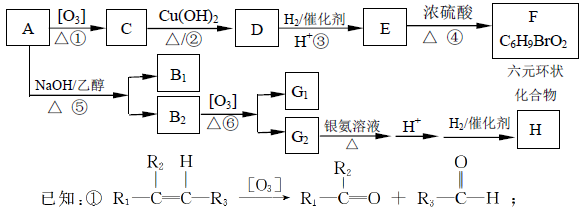
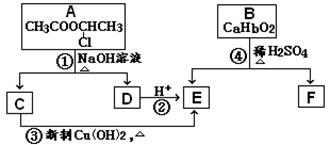
A��һ�־��ô��ᵯ����Ҫ�ɷ�,H���Ƶø߾���PLA����PLAΪ���Ͽ��Ƴ����̶��ݶ������ڹ��۽Ӻ�������������A ����Է�������Ϊ161��������C��H Ԫ���⣬��������һ��±��Ԫ�أ�������ֻ����һ������������A��H ��ת����ϵ����ͼ��ʾ����������������Cu(OH)2 ����Һ��1 mol C ��Ӧ������1 mol Cu2O ��1 mol D��B1��B2 ��Ϊͬ���칹�壬B1 ��Ħ������80g/mol��G1 ��G2 ��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1����
��һ��̼ԭ������������̼̼˫���Ľṹ(-C��C��C-)���ȶ���
������������⣺
(1) A �й����ŵ�����������������������������������B1 �ķ���ʽΪ����������������������
(2)�١���Ӧ��������ȥ��Ӧ����_________��A �Ľṹ��ʽ��������������������������������
(3)��Ӧ�ܵĻ�ѧ����ʽ����������������������������������������������������������
(4)д��C ������Cu(OH)2����Һ ��Ӧ�ķ���ʽ��������������������������������������������
(5)д��H��һ�����������ɸ߾���ķ�Ӧ����ʽ������������ ������������������������
(6)��������������E ��ͬ���칹�干�С����������������������������֡�
�ٺ�����������������NaHCO3��Ӧ���ۣ�OH����Br������ͬһ��̼ԭ���ϡ�
(7)���ʵ����C �������еķ��������� ��

��һ��̼ԭ������������̼̼˫���Ľṹ(-C��C��C-)���ȶ���
������������⣺
(1) A �й����ŵ�����������������������������������B1 �ķ���ʽΪ����������������������
(2)�١���Ӧ��������ȥ��Ӧ����_________��A �Ľṹ��ʽ��������������������������������
(3)��Ӧ�ܵĻ�ѧ����ʽ����������������������������������������������������������
(4)д��C ������Cu(OH)2����Һ ��Ӧ�ķ���ʽ��������������������������������������������
(5)д��H��һ�����������ɸ߾���ķ�Ӧ����ʽ������������ ������������������������
(6)��������������E ��ͬ���칹�干�С����������������������������֡�
�ٺ�����������������NaHCO3��Ӧ���ۣ�OH����Br������ͬһ��̼ԭ���ϡ�
(7)���ʵ����C �������еķ��������� ��
��1��̼̼˫������ԭ�� C6H8 ��2��5 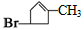
��3��HOOCCH2CHBrCH2CH(OH)CH3
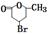 +H2O
+H2O
(4)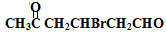 +2Cu(OH)2+NaOH
+2Cu(OH)2+NaOH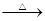
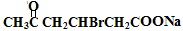 +Cu2O��+3H2O
+Cu2O��+3H2O
(5)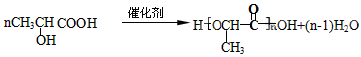 (6) 2
(6) 2
(7)ȡ����C��������������ˮ��Һ����ˮ�⣬Ȼ��������ữ���ټ�����������Һ������������ɫ��������˵��������ԭ��

��3��HOOCCH2CHBrCH2CH(OH)CH3

 +H2O
+H2O(4)
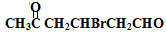 +2Cu(OH)2+NaOH
+2Cu(OH)2+NaOH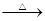
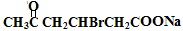 +Cu2O��+3H2O
+Cu2O��+3H2O(5)
 (6) 2
(6) 2(7)ȡ����C��������������ˮ��Һ����ˮ�⣬Ȼ��������ữ���ټ�����������Һ������������ɫ��������˵��������ԭ��
�����������ͼ��ת����ϵ�����нṹ��Ϣ֪��A�ķ���ʽΪC6H9Br�������������IJ���ֻ��һ�֣���AӦ�ǻ�״���������ӽṹ��ֻ��һ��-CH3����AΪ��������Ԫ��״�����A�ij�������IJ���C��Cu��OH��2��Ӧ�ı�����ϵ֪C������ֻ��һ��ȩ������A�м��������е�һ��������̼ԭ���ϣ��ٽ����Ŀ���ṩ�Ľṹ��Ϣ֪��A�ṹΪ
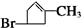 ������������Ϣ��֪A��������������C��CΪOHCCH2CHBrCH2COCH3��C����ΪD��ֻ����1��ȩ����DΪHOOCCH2CHBrCH2COCH3��D�ӳɺ�����E��EΪHOOCCH2CHBrCH2CHOHCH3��E����������Ӧ����F����FΪ
������������Ϣ��֪A��������������C��CΪOHCCH2CHBrCH2COCH3��C����ΪD��ֻ����1��ȩ����DΪHOOCCH2CHBrCH2COCH3��D�ӳɺ�����E��EΪHOOCCH2CHBrCH2CHOHCH3��E����������Ӧ����F����FΪ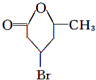 ��B1��A����ȥ��������ʽΪC6H8����Է�������Ϊ80��B1��B2 ��Ϊͬ���칹�壬B2������������Ӧ������G1 ��G2 ��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1�������B1��B2�Ľṹ��ʽΪ
��B1��A����ȥ��������ʽΪC6H8����Է�������Ϊ80��B1��B2 ��Ϊͬ���칹�壬B2������������Ӧ������G1 ��G2 ��Ϊͬ���칹�壬���ߵĺ˴Ź�������ֻ�����������G1�������B1��B2�Ľṹ��ʽΪ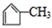 ��
��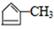 ��G1 ��G2�Ľṹ��ʽ�ֱ�ΪOHCCH2CHO��OHCCOCH3��G2ͨ��������Ӧ���ữ���ӳɺ�õ�H����H�Ľṹ��ʽΪHOOCCHOHCH3����
��G1 ��G2�Ľṹ��ʽ�ֱ�ΪOHCCH2CHO��OHCCOCH3��G2ͨ��������Ӧ���ữ���ӳɺ�õ�H����H�Ľṹ��ʽΪHOOCCHOHCH3������1���������Ϸ�����֪A �й����ŵ�����̼̼˫������ԭ�ӣ�B1 �ķ���ʽΪC6H8��
��2��A���������ƴ���Һ�з�����ȥ����١���Ӧ��������ȥ��Ӧ���Ǣݣ�A �Ľṹ��ʽΪ
 ��
����3����Ӧ����������Ӧ����ѧ����ʽΪHOOCCH2CHBrCH2CH(OH)CH3

 +H2O��
+H2O����4��C�к���ȩ�����ڼ�����������������ͭ����Һ��Ӧ�������ᣬ��Ӧ����ʽΪOHCCH2CHBrCH2COCH3��2Cu��OH��2��NaOH
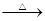 NaOOCCH2CHBrCH2COCH3��Cu2O����3H2O��
NaOOCCH2CHBrCH2COCH3��Cu2O����3H2O����5��H�����к����ǻ����Ȼ�����һ�����������ɸ߾���䷴Ӧ����ʽΪ
 ��
����6���ٺ�����������������NaHCO3��Ӧ��˵�������Ȼ����ۣ�OH����Br������ͬһ��̼ԭ���ϣ������������ͬ���칹��ṹ��ʽΪ
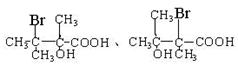 ��������2�֡�
��������2�֡���7��C�����е���ԭ�Ӳ���ֱ������������Һ��Ӧ����Ҫ��ͨ��ˮ�ⷴӦ�������ȷ�IJ�����ȡ����C��������������ˮ��Һ����ˮ�⣬Ȼ��������ữ���ټ�����������Һ������������ɫ��������˵��������ԭ�ӡ�

��ϰ��ϵ�д�
�����Ŀ

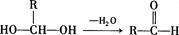
 CH2OH-CH2OH+2HCl ���Ա�ȩΪԭ����ȡ1,2-��������������еķ�Ӧ���������ǣ� ��
CH2OH-CH2OH+2HCl ���Ա�ȩΪԭ����ȡ1,2-��������������еķ�Ӧ���������ǣ� ��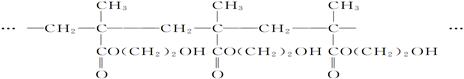


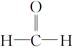 ��R��CH2��CHO�D��
��R��CH2��CHO�D��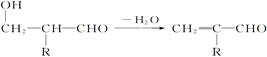
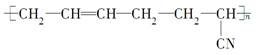 �������������͡����¹��ܣ��ϳɶ�����ԭ����(����)
�������������͡����¹��ܣ��ϳɶ�����ԭ����(����)