��Ŀ����
����9�֣�A��B��C��D��E�������ʵ���ɫ������ɫ(����ɫ�ܲ���)��A��B��ˮ��Ӧ��������ų���A��ˮ��Ӧ�ų���������л�ԭ�ԣ�B��ˮ��Ӧ�ų���������������ԣ�ͬʱ��������ҺC.C��������CO2��Ӧ����D���������CO2��Ӧ����E.E����������D.���ƶϣ�
��A��_____________��B��_____________��C��_____________��D��_____________��E��_____________(д��ѧʽ)
�ư�Ҫ��д���йط�Ӧ�Ļ�ѧ����ʽ��
B��ˮ��Ӧ�Ļ�ѧ����ʽ
E��������D�Ļ�ѧ����ʽ
��9�֣���A��K B��K2O2 C��KOH D��K2CO3 E��KHCO3��5�֣�
��2K2O2+2H2O��4KOH+O2�� 2KHCO3 K2CO3+CO2��+H2O��4�֣�
K2CO3+CO2��+H2O��4�֣�
����

��ϰ��ϵ�д�
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
�����Ŀ
ʵ����������������������Ҵ��Ʊ�1��2-�������飨BrCH2CH2Br����
��Ӧ���������У�
��һ�����Ҵ���Ũ���Ṳ�ȵ�170��������ϩ��CH3CH2OH
CH2=CH2��+H2O����
�ڶ�������ϩ��������1��2-�������飨CH2=CH2+Br2��BrCH2CH2Br����
���ܴ��ڵĸ���Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѣ�C2H5-O-C2H5�����¶ȹ��ߣ��Ҵ���Ũ���ᷴӦ����SO2��CO2���������壮
�й������б����£�
��Ӧװ����ͼ����ȥ���ȵ�װ�ã���
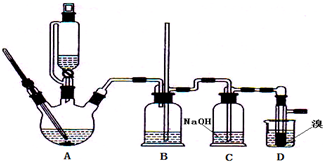
�ش��������⣺
��1���۷е㣺�Ҵ������ѣ��������ʽṹ�����֪ʶ����ԭ�� ��
��2����װ����������A��B��Cװ�������ԵIJ����� ��
��3�����D�е����ܷ��������¹ʣ�װ��B�п��ܹ۲쵽�������� ��
��4��װ��C�п��ܷ�����Ӧ�Ļ�ѧ����ʽ�� ��д��һ�����ɣ���
��5����1��2-��������ֲ�Ʒ���ڷ�Һ©���У���ˮ�����ã�����Ӧ�� �㣨��ϡ������¡�������������������δ��Ӧ��Br2������� ϴ�ӳ�ȥ������ĸ����
a��ˮ b������������Һ c���Ҵ�
��6�������������������������ѣ��ɲ��õij��ӷ����� ��
��7����Ӧʱװ��D���ձ���Ӧװ ���a����b��������װ��һ�ֵ�ԭ���� ��
a����ˮ b����ˮ��
��Ӧ���������У�
��һ�����Ҵ���Ũ���Ṳ�ȵ�170��������ϩ��CH3CH2OH
| H2SO4(Ũ) |
| 170�� |
�ڶ�������ϩ��������1��2-�������飨CH2=CH2+Br2��BrCH2CH2Br����
���ܴ��ڵĸ���Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѣ�C2H5-O-C2H5�����¶ȹ��ߣ��Ҵ���Ũ���ᷴӦ����SO2��CO2���������壮
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | �� | |
| ɫ��̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | �����ɫҺ�� |
| �ܶ�/��g?cm-3�� | 0.79 | 2.2 | 0.71 | 3.1 |
| �е�/�� | 78.5 | 132 | 34.6 | 59.47 |
| �۵�/�� | -130 | 9 | -116 | -7.25 |

�ش��������⣺
��1���۷е㣺�Ҵ������ѣ��������ʽṹ�����֪ʶ����ԭ��
��2����װ����������A��B��Cװ�������ԵIJ�����
��3�����D�е����ܷ��������¹ʣ�װ��B�п��ܹ۲쵽��������
��4��װ��C�п��ܷ�����Ӧ�Ļ�ѧ����ʽ��
��5����1��2-��������ֲ�Ʒ���ڷ�Һ©���У���ˮ�����ã�����Ӧ��
a��ˮ b������������Һ c���Ҵ�
��6�������������������������ѣ��ɲ��õij��ӷ�����
��7����Ӧʱװ��D���ձ���Ӧװ
a����ˮ b����ˮ��