��Ŀ����
��2011?������ģ�⣩����ѡ���У��������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
������A����������м��������൱����������м�ˮ�������Ӻ���������ӵ����ʵ����Dz���ģ�
B��������Һ�е������غ����ش��жϣ�
C��С�մ���Һ������������Һ�������Ϻ�����Ӧ���ɵ���̼������Һ��
D�����Ȼ����Һ�м���һ������ˮ��������Һ��ʾ���ԣ���ˮ�ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�
B��������Һ�е������غ����ش��жϣ�
C��С�մ���Һ������������Һ�������Ϻ�����Ӧ���ɵ���̼������Һ��
D�����Ȼ����Һ�м���һ������ˮ��������Һ��ʾ���ԣ���ˮ�ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�
����⣺A����������м������ᣬ�����Ӻ���������ӵ����ʵ����Dz���ģ�������Һ�������һ���ģ�
����c��K+��=c��NO3-������A��ȷ��
B��1.0mol/LK2CO3��Һ�е������غ�Ϊ��c��OH-��=c��HCO3-��+c��H+��+2c��H2CO3������B����
C��С�մ���Һ������������Һ�������Ϻ�����Ӧ���ɵ���̼������Һ�����ڵ���غ㣺��Na+��+c��H+��=2c��CO32-��+c��OH-��+c��HCO3-������C����
D�����Ȼ����Һ�м���һ������ˮ��������Һ��ʾ���ԣ���ˮ�ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�
c��NH4+����c��Cl-����c��OH-����c��H+������D����
��ѡA��
����c��K+��=c��NO3-������A��ȷ��
B��1.0mol/LK2CO3��Һ�е������غ�Ϊ��c��OH-��=c��HCO3-��+c��H+��+2c��H2CO3������B����
C��С�մ���Һ������������Һ�������Ϻ�����Ӧ���ɵ���̼������Һ�����ڵ���غ㣺��Na+��+c��H+��=2c��CO32-��+c��OH-��+c��HCO3-������C����
D�����Ȼ����Һ�м���һ������ˮ��������Һ��ʾ���ԣ���ˮ�ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�
c��NH4+����c��Cl-����c��OH-����c��H+������D����
��ѡA��
���������⿼��ѧ����Һ������Ũ��֮����غ��ϵ�Լ����ӹ���֪ʶ������ѧ�������ͽ��������������Ѷȴ�

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ
 ��2011?������ģ�⣩һ�������£���һ������A��B��C��D�������ʣ������ܱ������з������·�Ӧ��
��2011?������ģ�⣩һ�������£���һ������A��B��C��D�������ʣ������ܱ������з������·�Ӧ�� p C��g��+q D��g���ﵽƽ����B��Ũ��Ϊ0.5
p C��g��+q D��g���ﵽƽ����B��Ũ��Ϊ0.5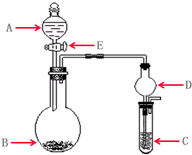 ��2011?������ģ�⣩ʵ������Ҫ����ijЩ����ʱ��ͨ��ʹ�ÿ��ٵķ����Ʊ������м���ʵ��ɿ�����ȡʵ����������������壬�������������ʵ�飮��ʵ��װ����ͼ��ʾ��
��2011?������ģ�⣩ʵ������Ҫ����ijЩ����ʱ��ͨ��ʹ�ÿ��ٵķ����Ʊ������м���ʵ��ɿ�����ȡʵ����������������壬�������������ʵ�飮��ʵ��װ����ͼ��ʾ��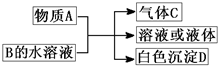 ��2011?������ģ�⣩A��B��C��D��Ϊ��ѧ��ѧ�������ʣ�����֮��ķ�Ӧ��ϵ��ͼ��ʾ��
��2011?������ģ�⣩A��B��C��D��Ϊ��ѧ��ѧ�������ʣ�����֮��ķ�Ӧ��ϵ��ͼ��ʾ��