��Ŀ����
��������Դ�ı��⣬�ú�ˮ������ʳ�κͽ���þ��þ�Ļ�����������������ͼ��ʾ��

��ش��������⣺
��1������ܷ�Ӧ�����ӷ���ʽ______��
��2��ʵ�����ɴ��νᾧ�ƾ��εIJ��������ܽ⡢���ˡ������Ȳ��衣
�ٴ����ᴿ�����й��˵�Ŀ����______������ţ���
A�����NaCl���� B����ȥ����������
C����ȥ�ӷ����� D����ȥ����������
�ڹ�����Ҫ�õ��IJ��������в�������______��______��
��3��Mg(OH)2���ȷֽ�����MgO��H2O��MgO�Ǹ��۵�Ļ����ijЩ��������Mg(OH)2��������ȼ�ԡ�����Mg(OH)2�ܹ���ȼ��ԭ��
��______����______��
��4�����MgCl2�ƽ���Mg�ķ�Ӧ����ʽΪ��MgCl2 Mg+Cl2�������0.2mol MgCl2�����ɽ���Mg������Ϊ______��ͬʱ�ɵõ����������Ϊ______L(��״��)��
Mg+Cl2�������0.2mol MgCl2�����ɽ���Mg������Ϊ______��ͬʱ�ɵõ����������Ϊ______L(��״��)��
��1��Mg(OH)2+2H+==Mg2++2H2O
��2����B ��©�� �ձ�
��3��Mg(OH)2���ȷֽ�����MgO�����︲���ڿ�ȼ�������ֹ�˿�ȼ���ȼ�շֽ����ɵ�ˮ�ή�ͻ������¶�
��4��4.8g 4.48L
��4�����0.2mol MgCl2��������0.2 Mg��������Ϊ4.48g�����������ʵ���Ϊ0.2mol�����״���µ����Ϊ4.48L��
��2����B ��©�� �ձ�
��3��Mg(OH)2���ȷֽ�����MgO�����︲���ڿ�ȼ�������ֹ�˿�ȼ���ȼ�շֽ����ɵ�ˮ�ή�ͻ������¶�
��4��4.8g 4.48L
��4�����0.2mol MgCl2��������0.2 Mg��������Ϊ4.48g�����������ʵ���Ϊ0.2mol�����״���µ����Ϊ4.48L��

��ϰ��ϵ�д�
�����Ŀ
 ��2009?�Ϻ�����������Դ�ı��⣬�̲��ŷḻ�Ļ�ѧԪ�أ����ȡ��塢��ȣ�
��2009?�Ϻ�����������Դ�ı��⣬�̲��ŷḻ�Ļ�ѧԪ�أ����ȡ��塢��ȣ�

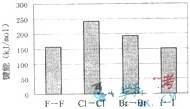
 (4)±�ص��ʵļ��ܴ�С����ͼ����ͼ�ƶϣ�
(4)±�ص��ʵļ��ܴ�С����ͼ����ͼ�ƶϣ� ����ʽΪ ����Ӧ���б��ƻ��Ļ�ѧ����
����ʽΪ ����Ӧ���б��ƻ��Ļ�ѧ���� �� ��(����ԡ��Ǽ��ԡ�)��
�� ��(����ԡ��Ǽ��ԡ�)�� λ�����ڱ��ĵ�
λ�����ڱ��ĵ�  3)±�ص��ʼ���
3)±�ص��ʼ��� ���������������϶������ŵݱ���ɡ������й�˵����ȷ���� ��
���������������϶������ŵݱ���ɡ������й�˵����ȷ���� �� ��Ļ�ԭ��HF��HCl��HBr��HI��˳�����μ���
��Ļ�ԭ��HF��HCl��HBr��HI��˳�����μ���