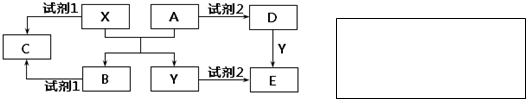
��Ŀ����
��ͼ��Ԫ�����ڱ��Ŀ�ܣ�����Ԫ�����ڱ��ش��������⣺
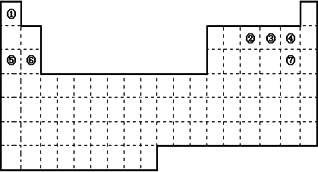
(1)���ڱ��е�Ԫ�آݺ�Ԫ�آ�����������ˮ�������ǿ��˳����________(�û�ѧʽ��ʾ)�����ڱ��е�Ԫ�آܺ�Ԫ�آߵ��⻯��ķе�ߵ�˳����________(�û�ѧʽ��ʾ)��
(2)��д���ڵ��⻯��ĵ���ʽ________���۵��⻯��Ľṹʽ________��
(3)�١���Ԫ�صĵ��ʣ��ڳ����»�ѧ�����ȶ���ͨ������������������________(��д����ʽ)��
(4)��д����ҵ���Ʊ��ߵ��ʵĻ�ѧ����ʽ________��
�𰸣�
������
������
|
����(1)NaOH��Mg(OH)2��HF��HCl�� ����(2) ����(3)N2�� ����(4)2NaCl��2H2O��2NaOH��H2����Cl2��(������ͨ��)�� |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ