��Ŀ����
��NaHCO3��KHCO3��ɵĻ����ֱ�������ͬŨ�ȵ�������з�Ӧ������������£�
| 100mL���� | 100mL���� | 100mL���� | |
| m������ | 9.2g | 15.7g | 27.6g |
| V��CO2������״���� | 2.24L | 3.36L | 3.36L |
- A.�������ʵ���Ũ��Ϊ3.0 mol/L
- B.15.7 g����������ᷴӦ����ʣ��
- C.����9.2 g�����ʱ����HCl 0.1 mol
- D.�������NaHCO3��KHCO3��������Ϊ1��1
AD
�������������֪��9.2 g�������100 mL���ᷴӦʱ�����������27.6 g�������100 mL��������ʱ����㣻����3.36L������̼��Ҫ����������Ϊ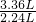 ��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
A���ڶ���ʵ��������㣬�������ʣ�࣬�ڶ���ʵ���з�����ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ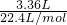 =0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2�����ݴ˼��㣻
=0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2�����ݴ˼��㣻
B����������3.36L������̼��Ҫ��������������15.7g�Ƚ��жϣ�
C�����ڵڶ���ʵ������������ڵ�һ��ʵ�飬������ͬ����˵����������9.2 gʱ����������������ɶ�����̼����������������ʵ�����
D����һ��ʵ���л������ȫ��Ӧ�����������������ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ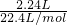 =0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪n��CO2��=n��NaHCO3��+n��KHCO3���������û���������з��̼���x��y��ֵ����������������NaHCO3��KHCO3�������ȣ�
=0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪n��CO2��=n��NaHCO3��+n��KHCO3���������û���������з��̼���x��y��ֵ����������������NaHCO3��KHCO3�������ȣ�
����������֪��9.2 g�������100 mL���ᷴӦʱ�����������27.6 g�������100 mL��������ʱ����㣻����3.36L������̼��Ҫ����������Ϊ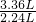 ��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
A���ڶ���ʵ��������㣬�������ʣ�࣬�ڶ���ʵ���з�����ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ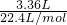 =0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2��=0.15mol����Ԫ�ص����ʵ���Ũ��Ϊ
=0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2��=0.15mol����Ԫ�ص����ʵ���Ũ��Ϊ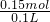 =1.5mol/L����A����
=1.5mol/L����A����
B������3.36L������̼��Ҫ����������Ϊ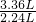 ��9.2g=13.8g��15.7�ˣ����Ի�������������㣬��B��ȷ��
��9.2g=13.8g��15.7�ˣ����Ի�������������㣬��B��ȷ��
C�����ڵڶ���ʵ������������ڵ�һ��ʵ�飬������ͬ����˵����������9.2 gʱ������������ɶ�����̼�����ʵ���Ϊ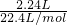 =0.1mol��������ӦH++HCO3-=CO2��+H2O���ɷ���ʽ��֪�����ĵ��������ʵ���Ϊ0.1mol����C��ȷ��
=0.1mol��������ӦH++HCO3-=CO2��+H2O���ɷ���ʽ��֪�����ĵ��������ʵ���Ϊ0.1mol����C��ȷ��
D����һ��ʵ���л������ȫ��Ӧ�����������������ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ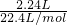 =0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪��x+y=0.1mol�����������Ϊ9.2g������84x+100y=9.2g���������̽��x=0.05 mol��y=0.05 mol���ʻ������NaHCO3��KHCO3��������Ϊ84��100=21��25����D����
=0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪��x+y=0.1mol�����������Ϊ9.2g������84x+100y=9.2g���������̽��x=0.05 mol��y=0.05 mol���ʻ������NaHCO3��KHCO3��������Ϊ84��100=21��25����D����
��ѡAD��
���������⿼��������йؼ��㣬��Ŀ�ѶȽϴ���ע��Ƚ��������ݣ��жϷ�Ӧ�ij̶ȣ�Ϊ������Ĺؼ��㣬Ҳ���״��㣮
�������������֪��9.2 g�������100 mL���ᷴӦʱ�����������27.6 g�������100 mL��������ʱ����㣻����3.36L������̼��Ҫ����������Ϊ
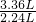 ��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮A���ڶ���ʵ��������㣬�������ʣ�࣬�ڶ���ʵ���з�����ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ
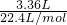 =0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2�����ݴ˼��㣻
=0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2�����ݴ˼��㣻B����������3.36L������̼��Ҫ��������������15.7g�Ƚ��жϣ�
C�����ڵڶ���ʵ������������ڵ�һ��ʵ�飬������ͬ����˵����������9.2 gʱ����������������ɶ�����̼����������������ʵ�����
D����һ��ʵ���л������ȫ��Ӧ�����������������ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ
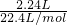 =0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪n��CO2��=n��NaHCO3��+n��KHCO3���������û���������з��̼���x��y��ֵ����������������NaHCO3��KHCO3�������ȣ�
=0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪n��CO2��=n��NaHCO3��+n��KHCO3���������û���������з��̼���x��y��ֵ����������������NaHCO3��KHCO3�������ȣ�����������֪��9.2 g�������100 mL���ᷴӦʱ�����������27.6 g�������100 mL��������ʱ����㣻����3.36L������̼��Ҫ����������Ϊ
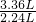 ��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮
��9.2g=13.8g�����Եڶ���ʵ�����β��㣬�������ʣ�࣮A���ڶ���ʵ��������㣬�������ʣ�࣬�ڶ���ʵ���з�����ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ
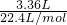 =0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2��=0.15mol����Ԫ�ص����ʵ���Ũ��Ϊ
=0.15mol���ɷ���ʽ��֪n��HCl��=n��CO2��=0.15mol����Ԫ�ص����ʵ���Ũ��Ϊ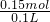 =1.5mol/L����A����
=1.5mol/L����A����B������3.36L������̼��Ҫ����������Ϊ
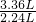 ��9.2g=13.8g��15.7�ˣ����Ի�������������㣬��B��ȷ��
��9.2g=13.8g��15.7�ˣ����Ի�������������㣬��B��ȷ��C�����ڵڶ���ʵ������������ڵ�һ��ʵ�飬������ͬ����˵����������9.2 gʱ������������ɶ�����̼�����ʵ���Ϊ
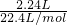 =0.1mol��������ӦH++HCO3-=CO2��+H2O���ɷ���ʽ��֪�����ĵ��������ʵ���Ϊ0.1mol����C��ȷ��
=0.1mol��������ӦH++HCO3-=CO2��+H2O���ɷ���ʽ��֪�����ĵ��������ʵ���Ϊ0.1mol����C��ȷ��D����һ��ʵ���л������ȫ��Ӧ�����������������ӦH++HCO3-=CO2��+H2O�����ɶ�����̼�����ʵ���Ϊ
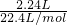 =0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪��x+y=0.1mol�����������Ϊ9.2g������84x+100y=9.2g���������̽��x=0.05 mol��y=0.05 mol���ʻ������NaHCO3��KHCO3��������Ϊ84��100=21��25����D����
=0.1mol����9.2 g�������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol������̼Ԫ���غ��֪��x+y=0.1mol�����������Ϊ9.2g������84x+100y=9.2g���������̽��x=0.05 mol��y=0.05 mol���ʻ������NaHCO3��KHCO3��������Ϊ84��100=21��25����D������ѡAD��
���������⿼��������йؼ��㣬��Ŀ�ѶȽϴ���ע��Ƚ��������ݣ��жϷ�Ӧ�ij̶ȣ�Ϊ������Ĺؼ��㣬Ҳ���״��㣮

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
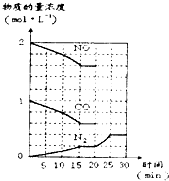
 CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ�
CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ� CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ�
CO2��SO2��NOx����Ҫ�ķǽ�������������ǵ����滷��ϢϢ��أ�