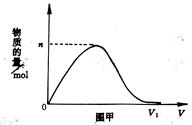
��Ŀ����
��10�֣�Ԫ���ȼ��仯����������������������й㷺��Ӧ�á�
��25��ʱ��PbCl2�����ڲ�ͬŨ������(mol��L��1)�е��ܽ��(mmol��L��1)��ͼ��
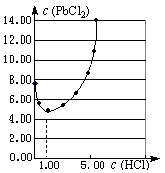
�����Ʊ�PbCl2��ʵ���У�ϴ��PbCl2�������ѡ�� ��
a������ˮ b��1mol��L��1����
c��5 mol��L��1���� d��10mol��L��1����
�ڵ������Ũ��С��1mol��L��1ʱ����������Ũ�ȵ�����PbCl2 ���ܽ�ȼ�С����ԭ���� ��
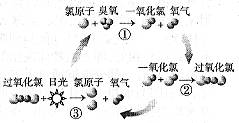
��TCCA�㷺����Ư�ס�ɱ����������ѧ��Ϊ���Ⱦ����-2,4,6-��ͪ������ʽΪ��C3Cl3N3O3��
��TCCA���Ӿ�����ȫ�ԳƵĽṹ��������һ����Ԫ��������ṹ��ʽΪ ��
��ʹ��TCCAʱ�����Ƚ��������ܽ���ˮ����ˮ�����֮һΪC3H3N3O3����һ�ֲ�
�����ǿ�����ԣ��ܹ�ɱ��������д����һ�ֲ���ĵ���ʽ ��
�Ǹ������(AP)��Ϊһ�������Ĺ����ƽ��������ڵ����ͻ�����䡣Ŀǰ����Ϊ�Ƚ����Ʊ������ǵ��ߴ�������õ��ߴ������ᣬ����ߴ�������������Ӧ�Ƴɸ�����李�д���ɴ��������Ʊ��������������Ӧʽ�� ��
��25��ʱ��PbCl2�����ڲ�ͬŨ������(mol��L��1)�е��ܽ��(mmol��L��1)��ͼ��

�����Ʊ�PbCl2��ʵ���У�ϴ��PbCl2�������ѡ�� ��
a������ˮ b��1mol��L��1����
c��5 mol��L��1���� d��10mol��L��1����
�ڵ������Ũ��С��1mol��L��1ʱ����������Ũ�ȵ�����PbCl2 ���ܽ�ȼ�С����ԭ���� ��
��TCCA�㷺����Ư�ס�ɱ����������ѧ��Ϊ���Ⱦ����-2,4,6-��ͪ������ʽΪ��C3Cl3N3O3��
��TCCA���Ӿ�����ȫ�ԳƵĽṹ��������һ����Ԫ��������ṹ��ʽΪ ��
��ʹ��TCCAʱ�����Ƚ��������ܽ���ˮ����ˮ�����֮һΪC3H3N3O3����һ�ֲ�
�����ǿ�����ԣ��ܹ�ɱ��������д����һ�ֲ���ĵ���ʽ ��
�Ǹ������(AP)��Ϊһ�������Ĺ����ƽ��������ڵ����ͻ�����䡣Ŀǰ����Ϊ�Ƚ����Ʊ������ǵ��ߴ�������õ��ߴ������ᣬ����ߴ�������������Ӧ�Ƴɸ�����李�д���ɴ��������Ʊ��������������Ӧʽ�� ��
�Ţ�b ��Cl��Ũ������PbCl2���ܽ�ƽ�������ƶ�
�Ƣ� ��
��
�Ǣ�HClO+3H2O��6e����ClO4��+7H+
�Ƣ�
 ��
��
�Ǣ�HClO+3H2O��6e����ClO4��+7H+
��

��ϰ��ϵ�д�
�����Ŀ

 CO2+H2
CO2+H2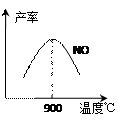 ��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��
��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��