��Ŀ����
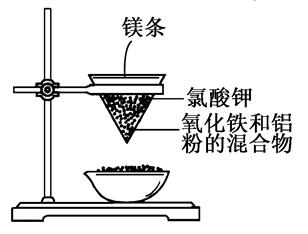
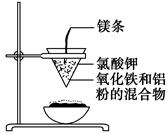
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�����������������������������ʡ�����ʵ�鲽������ͼ��ʾ��
��������ͼʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500mLϡ��ˮ��ÿ������39.20g������ҪȡŨ��ˮ��ÿ������251.28g����__________mL���ù��Ϊ_______mL��Ͳ��ȡ��
��2����ˮ������õ���������ϵ�����ˣ���Һ�г�K����SO42���⣬���д�����NH4��������NH4���ķ�����______________________________________________��
��3��д�����������������ʵĻ�ѧʽ________________________________________��
��4����ҺI�ijɷ���ˮ��______________��
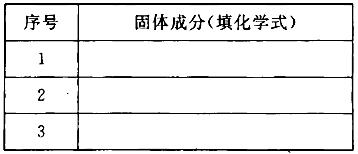
��5��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ��������������в��裺
�ٳ�ȡ�ص�������������ˮ����������______��Һ��������ɫ������
��___________��__________��_________(������дʵ���������)��
����ȴ�����ء�
��6��������Ϊmg�����������ʵ���Ϊnmol����������K2SO4�����ʵ���Ϊ��___________mol���ú���m��n�Ĵ���ʽ��ʾ����
��1��78 ��100
��2��ȡ��Һ����������NaOH�����ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ���������������֣�
��3��Al(OH)3��Al2O3��Fe2O3
��4��K2SO4��(NH4)2SO4
��5��BaCl2�����ˣ�ϴ�ӣ�����
��6��
�����������������1������500mLϡ��ˮ��ÿ������39.20g��������Ҫʹ��500mL����ƿ����ҪŨ��ˮ��ÿ������250.28g���������Ϊ�� ��0.078L=78mL���ʴ�Ϊ��78��100��
��0.078L=78mL���ʴ�Ϊ��78��100��
��2������ʹ���������ƺͺ�ɫʯ����ֽ���м��飬�������NH4���ķ���Ϊ��ȡ��Һ����������NaOH���ȣ����ɵ�������ʹ��ʪ�ĺ�ɫʯ����ֽ������
��3������ʯ����ɺ��������ƣ������ܹ��백ˮ��Ӧ���������������������������������������������ʲ��백ˮ��ӦҲ������ˮ�����Գ�����Ϊ��Al(OH)3��Al2O3��Fe2O3��
��4������ʯ����ɺ��������ƣ����ݹ�������ת����ϵ��֪����ҺI�к���K2SO4�ͷ�Ӧ����(NH4)2SO4��
��5����Ϸ����к���K2SO4��(NH4)2SO4��Ҫ������ɫ�������������ҺΪBaCl2��Һ��Ȼ����в������Ƚ����Һ���ˣ�Ȼ��ϴ�ӳ������������ȴ����أ�
��6������Ϊmg����174n��K2SO4��+132n[(NH4)2SO4]=m�����������ʵ���Ϊnmol����BaSO4�����ʵ���Ϊnmol������������غ��У�n��K2SO4��+n[(NH4)2SO4]=n��������ã�n��K2SO4��= mol��
mol��
���㣺���鹤�����̵����⡢���ӵļ����빲�桢���ʷ����ᴿ�Ȼ������������������뻯ѧ�����ѧ����ȡ�


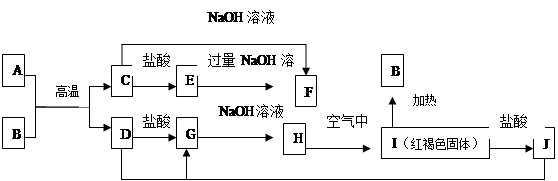

 B(��ˮ��Һ�н���)
B(��ˮ��Һ�н���)