��Ŀ����
��16�֣�ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
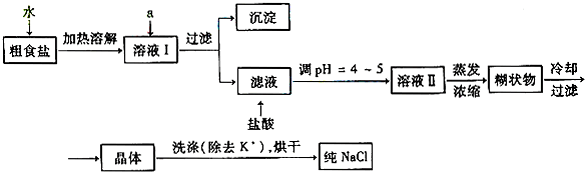
(1)��ʳ�γ�������K����Ca2����Mg2����Fe3����SO42�����������ӣ�ʵ�����ᴿNaCl���������£�
�ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ �� 75%�Ҵ������Ȼ�̼������ȥ��ҺI�е�Ca2����Mg2����Fe3����SO42�����ӣ�ѡ��a���������Լ������μ�˳�������� ��ֻ�ѧʽ����
(2)���ᴿ��NaCl����500 mL 4.00 mol��L-1 NaCl��Һ������������ҩ�ס���������������ƽ���ձ�����Ҫ �����������ƣ���������ʱ���ӿ̶��ߣ���������Һ��Ũ�� ���ƫ����ƫС������Ӱ�족����
(3)�ڵ�ⱥ��ʳ��ˮ��ʵ���У����ռ���H2Ϊ3.36 L���ڱ�״���£�����ת�Ƶ��ӵĸ���Ϊ ��ͬ���������ռ���Cl2 �����������=��������3.36 L��
(4)ʵ�����Ʊ�Cl2ͨ�����ö���������Ũ���Ṳ������ȡ��������Ӧ�����ӷ���ʽΪ�� ��
�ݴˣ���������������װ����ѡ���Ʊ����ռ��������Cl2��װ�� ������ţ�
��ѡ���Ʊ������װ�ã�
��ѡװ���е�����ϴ��ƿ��Ӧ����ʢװ �� �����Լ������ƣ������ϻ�ѧƽ���ƶ�ԭ�����͵�һ��ϴ��ƿ���Լ�ѡ������� ��β���� ��Һ���ѧʽ�����գ�д��������Ӧ�����ӷ���ʽ ��
��16�֣�
��1�� BaCl2��NaOH ��Na2CO3��2�֣���������˳��Ҳ���֣�
��2��500 mL������ƿ����ͷ�ιܣ�2�֣�д�벻д����Ͳ�������۷֣�
ƫС��1�֣�
��3��0.3NA��1.806��1023��1�� ��
< ��1�֣�
��4��MnO2��4H����2Cl�� Mn2����Cl2����2H2O��2�� ��
Mn2����Cl2����2H2O��2�� ��
d ��1�� ��
����ʳ��ˮ�͵��Ȼ�����Һ��Ũ���ᣨ2�� ��
Cl2����ˮ����ˮ������ӦCl2 +H2O  H++Cl��+HClO,����Cl����Ũ��ʹƽ�������ƶ����������ռ���������� ��2�� �� NaOH ��1�� �� Cl����2OH����Cl����ClO����H2O��2�� ��
H++Cl��+HClO,����Cl����Ũ��ʹƽ�������ƶ����������ռ���������� ��2�� �� NaOH ��1�� �� Cl����2OH����Cl����ClO����H2O��2�� ��
����

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�