��Ŀ����
����Ŀ�����������������dz��ḻ����ش��������⣺
��1����֪��![]()
![]()
![]()
![]() ��
��![]()
![]() _____________��
_____________��
��2��![]() �¶�ʱ���ݻ�Ϊ2L�ĺ����ܱ������г���
�¶�ʱ���ݻ�Ϊ2L�ĺ����ܱ������г���![]() ��
��![]() ������Ӧ��
������Ӧ��![]() ��
��![]() ��
��![]() ʱ�ﵽƽ�⣬��ʱ
ʱ�ﵽƽ�⣬��ʱ![]() ת����Ϊ80%��
ת����Ϊ80%��
��![]() �ڸ÷�Ӧ��ƽ������
�ڸ÷�Ӧ��ƽ������![]() _____________��
_____________��
��![]() �¶�ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��
�¶�ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��![]() ___________��
___________��
�������ı������������ƽ������������ͨ��![]() �����´ﵽƽ���
�����´ﵽƽ���![]() ����ϵ�еİٷֺ���_____________����������С�����䡱��
����ϵ�еİٷֺ���_____________����������С�����䡱��
��3����ҵ�ϳɰ��ķ�Ӧԭ��Ϊ��![]() ��
��![]() �����ڸ÷�Ӧ���ȿ������ƽ����ϵ��
�����ڸ÷�Ӧ���ȿ������ƽ����ϵ��![]() �İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ����______��������ţ�
�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ����______��������ţ�
a�������¶� b����ƽ����ϵ�еİ���������� c��������ϵѹǿ d��������ʵĴ���
��4����ͼ��ʾ��![]() �¶�ʱ��
�¶�ʱ��![]() ��
��![]() ��Ͷ�ϱ���ƽ��ʱ
��Ͷ�ϱ���ƽ��ʱ![]() ��������Ĺ�ϵ����������
��������Ĺ�ϵ����������![]() ��
��![]() ֮����ת����
֮����ת����

���������![]() ��
��![]() ��Ͷ�ϱȣ����������ƽ��ʱ
��Ͷ�ϱȣ����������ƽ��ʱ![]() ���������������ߵ�
���������������ߵ�![]() ʱ
ʱ![]() ��ƽ���������Ϊ_____________��
��ƽ���������Ϊ_____________��
���𰸡�![]()
![]() 80 ��� c 10%
80 ��� c 10%
��������
��1���ɸ�˹���ɿ����![]() ��
��
��2����![]() ���㡣
���㡣
��������ʽ�ɼ����ƽ��ʱ��![]() ,
,![]() �ɼ����
�ɼ����![]() ��
��
��ƽ����ٴγ���![]() ����Ч������ѹǿ��ƽ�������������С�������ƶ�����
����Ч������ѹǿ��ƽ�������������С�������ƶ�����![]() ����ϵ�зֺ������
����ϵ�зֺ������
��3�����ӷ�Ӧ���ʵĴ�ʩ�������¶ȡ�����ѹǿ������Ũ�ȡ�ʹ�ô�������Ҫ����ƽ����ϵ��H2��ת���ʣ�ƽ�������������ƶ��������������ԭ���жϸ�ѡ��ɣ�
��4��������֪����![]() ��ʱ��
��ʱ��![]() �����������0.85��
�����������0.85��![]() ��
��![]() �����������ռ0.15����
�����������ռ0.15����![]() ��
��![]() ��Ͷ�ϱ���2��1����
��Ͷ�ϱ���2��1����![]() �����������10%��
�����������10%��
��1���ɸ�˹���ɣ���֪����![]()
![]()
��![]()
![]() ��+�ڣ���2�ã�
��+�ڣ���2�ã�![]()
![]() ��
��
��2����![]() ��
��
��������ʽ��
2NO(g)��O2(g)=2NO2(g)
nʼ/mol 4 2
��n/mol 4��0.8 1.6 3.2
nƽ/mol 0.8 0.4 3.2
ƽ��ʱ��![]() ,
,![]() ��K=
��K=![]() �ɼ����
�ɼ����![]() ��
��
��ƽ����ٴγ���![]() ����Ч������ѹǿ��ƽ�������������С�������ƶ�����
����Ч������ѹǿ��ƽ�������������С�������ƶ�����![]() ����ϵ�зֺ������
����ϵ�зֺ������
��3��a�������¶ȷ�Ӧ��������ƽ�������ƶ��������H2��ת���ʣ���a����
b������NH3����С�������Ũ�ȣ���Ӧ���ʽ��ͣ���ƽ�������ƶ���H2��ת��������b����
c.����ѹǿ���ӷ�Ӧ����,ƽ�����������ƶ�������ƽ����ϵ��H2��ת���ʣ���c��ȷ��
d.���������Ӧ��������ƽ�ⲻ�ƶ�����d����
����������ѡ����c��
��4��������֪����![]() ��ʱ��
��ʱ��![]() �����������0.85��
�����������0.85��![]() ��
��![]() �����������ռ0.15����
�����������ռ0.15����![]() ��
��![]() ��Ͷ�ϱ���2��1����
��Ͷ�ϱ���2��1����![]() �����������10%��
�����������10%��

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�����Ŀ���������������IJ����������ŷŵ�NO2(N2O4)��CO��NO�������йء��ش��������⣺
��1���û���̿��β�����д������������·�Ӧ��
��Ӧa��C(s)��NO2(g)![]() 1/2N2(g)��CO2(g) ��H=-32.1kJ��mol1
1/2N2(g)��CO2(g) ��H=-32.1kJ��mol1
��Ӧb��2C(s)��N2O4(g)![]() N2(g)��2CO2(g) ��H=-28.2kJ��mol1
N2(g)��2CO2(g) ��H=-28.2kJ��mol1
��NO2����N2O4���Ȼ�ѧ����ʽΪ____��
��2���û���̿����β��ʱ���ɷ�����Ӧ��C(s)��2NO(g)![]() N2(g)��CO2(g) ��H=-34.0 kJ��mol1��������������T1���ò�ͬʱ�����NO��N2��Ũ�����£�
N2(g)��CO2(g) ��H=-34.0 kJ��mol1��������������T1���ò�ͬʱ�����NO��N2��Ũ�����£�
ʱ��/min Ũ��/mol��L1 ���� | 0 | 5 | 10 | 15 | 20 | 25 |
NO | 1.20 | 0.74 | 0.56 | 0.56 | 0.63 | 0.63 |
N2 | 0 | 0.23 | 0.32 | 0.32 | 0.36 | 0.36 /td> |
��15 min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣻�����ϱ������жϸı������������___(����ĸ)��
a���ʵ���С��������� b�����������Ļ���̿
c��ͨ��������NO d���ʵ������¶�
��0��10 min�ڣ�CO2��ƽ����Ӧ����v(CO2)=___��
��3����ij���ʵ�ƽ���ѹ���������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��������Kp�������ܱ������м���������C��һ������N2O4���壬ά���¶�T2�棬�ڲ�ͬѹǿ�·�����1���з�Ӧb��������ͬʱ��N2O4��ת������ѹǿ�仯��ͼ��ʾ��

��1.1��106 Paʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=___�����������λ��Ч���֡���֪�������ѹ(p��)=������ѹ(p��)�������������
��4����I2O5��������β���е�CO������I2O5(s)+5CO(g)![]() 5CO2(g)+I2(g) ��H=Q�����ݻ�Ϊ1 L���ܱ������г���5 mol CO��������I2O5ģ��÷�Ӧ��
5CO2(g)+I2(g) ��H=Q�����ݻ�Ϊ1 L���ܱ������г���5 mol CO��������I2O5ģ��÷�Ӧ��
�ٲ��CO��ƽ��ת�������¶ȱ仯�Ĺ�ϵ��ͼ������˵����ȷ����____(����ĸ)��

A��Q��0
B����X�㵽Y���ͨ��ͨ��COʵ��
C����Y�㵽Z���ͨ������ѹǿʵ��
D��600 Kʱ��Y��CO��v����v��
����֪Y�������Ϊ��600K��0.6�����ڴ�������I2(g)���������Ϊ___������ȷ��0.1%��
����Ŀ��(1)2017���п�Ժij�о��Ŷ�ͨ�����һ������Na-Fe3O4/HZSM-5��ܸ��ϴ������ɹ�ʵ����CO2ֱ�Ӽ�����ȡ����ֵ���ͣ����о��ɹ�������Ϊ��CO2��ת�������ͻ���Խ�չ����
��֪��H2(g)+1/2O2(g)=H2O(l) ��H1 = ��aKJ/mol
C8H18(1)+25/2O2(g)=8CO2(g)+9H2O(1) ��H2= ��bKJ/mol
��д��25����101kPa�����£�CO2��H2��Ӧ��������(��C8H18��ʾ)���Ȼ�ѧ����ʽ_________________________________��
(2)����CO2��H2Ϊԭ�ϣ��ں��ʵĴ���(��Cu/ZnO����)�����£�Ҳ�ɺϳ�CH3OH���漰�ķ�Ӧ�У�
�ף�CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H= �� 53.7kJ��mol-1 ƽ�ⳣ��K1
CH3OH(g)+H2O(g) ��H= �� 53.7kJ��mol-1 ƽ�ⳣ��K1
�ң�CO2(g)+H2(g) ![]() CO(g)+H2O(g) ��H= + 41.2kJ��mol-1 ƽ�ⳣ��K2
CO(g)+H2O(g) ��H= + 41.2kJ��mol-1 ƽ�ⳣ��K2
��CO(g)+2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��K=______(�ú�K1��K2�ı���ʽ��ʾ)���÷�Ӧ��H_____0(��������������С����)��
CH3OH(g)��ƽ�ⳣ��K=______(�ú�K1��K2�ı���ʽ��ʾ)���÷�Ӧ��H_____0(��������������С����)��
�����CO2ת��ΪCH3OHƽ��ת���ʵĴ�ʩ��___________(��д����)��
�۴����ͷ�Ӧ��ϵ�Ĺ�ϵ��������Կ�Ĺ�ϵһ�������и߶ȵ�ѡ���ԡ���������ʵ�飬����CO2��H2��ʼͶ�ϱȾ�Ϊ1��2.2��������ͬ��Ӧʱ��(t1min)��
�¶�(K) | ���� | CO2ת����(%) | �״�ѡ����(%) | �ۺ�ѡ�� |
543 | Cu/ZnO���װ����� | 12.3 | 42.3 | A |
543 | Cu/ZnO����Ƭ���� | 11.9 | 72.7 | B |
553 | Cu/ZnO���װ����� | 15.3 | 39.1 | C |
553 | Cu/ZnO����Ƭ���� | 12.0 | 70.6 | D |
�ɱ����е����ݿ�֪����ͬ�¶��²�ͬ�Ĵ�����CO2��ת��ΪCH3OH��ѡ����������Ӱ�죬�����ϱ��������ݽ�Ϸ�Ӧԭ������������ѡ��Ϊ___________(����ĸ����)��
(3)��CO��H2Ϊԭ�Ϻϳ��״��ķ�ӦΪ��CO(g)+2H2(g)![]() CH3OH(g)���������Ϊ2L�����������ܱ����������������У��ֱ���1molCO��2molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣��ͼΪ���������еķ�Ӧ�����е�5minʱH2���������ʾ��ͼ��������һ��������Ӧһ���ﵽƽ��״̬��
CH3OH(g)���������Ϊ2L�����������ܱ����������������У��ֱ���1molCO��2molH2�����������ķ�Ӧ�¶ȷֱ�ΪT1��T2��T3�Һ㶨���䡣��ͼΪ���������еķ�Ӧ�����е�5minʱH2���������ʾ��ͼ��������һ��������Ӧһ���ﵽƽ��״̬��
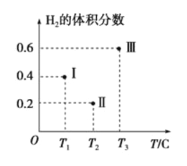
��0��5minʱ��������������CH3OH��ʾ�Ļ�ѧ��Ӧ����Ϊ_________________��
������������һ���ﵽƽ��״̬��������________(��д��������)��






