��Ŀ����
A��B��C��D���ֶ�����Ԫ�ص�ԭ��������������A��Dͬ�壬B��Cͬ���ڣ�A��B��ɵĻ������Ϊ��̬������A��Bԭ����֮��Ϊ4��1����A��C��ɵ����ֻ������Һͱ���ΪҺ̬������A��Cԭ����֮��Ϊ1��1������Ϊ2��1����D��C��ɵĻ����ﶡ���춼Ϊ��̬������D��Cԭ����֮��Ϊ1��1������Ϊ2��1��д����ѧʽ����________����________����________����________����________��д��BԪ�ص�������������������Ӧ�Ļ�ѧ����ʽ________��
�𰸣�
������
������
|
�ף�CH4,�ң�H2O2,����H2O,����Na2O2,�죺Na2O,2 Na2O 2+2 H2O =4 Na++4OH-+O2�� |

��ϰ��ϵ�д�
 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
A��B��C��D���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С��A��C�ĺ˵����֮��3��4��D�ֱ���A��B��C���γ�10�����ӵķ���X��Y��Z��������������ȷ���ǣ�������
| A��X��Y��Z���ַ��ӵ��ȶ������� | B����������Ԫ�ع����γ����ֵ��� | C��X��Y��Z���ַ��ӵķе������� | D��X��Y��Z���ַ��Ӿ�Ϊ���Է��� |

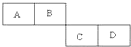 A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����
A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����