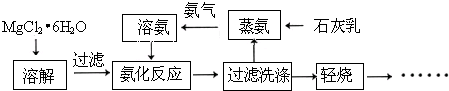
��Ŀ����
��������FeCl3��Һ�μӵķ�Ӧ�У���Fe3+ˮ���йصķ�Ӧ��
��FeCl3��Һ��Cu�ķ�Ӧ �ڽ�FeCl3��Һ�������ɣ����������յõ�Fe2O3 ��FeCl3��Һ��KI�ķ�Ӧ �ܱ���FeCl3��Һ�����ˮ���Ʊ�Fe(OH)3���� ��FeCl3��Һ��H2S�ķ�Ӧ��FeCl3��Һ��NaHCO3��Һ�ķ�Ӧ ������FeCl3��Һ�����һ����������
A�� �٢ܢ� B�� �ڢۢݢ� C�� �ڢܢޢ� D�� �٢ڢۢܢݢޢ�
���𰸡�
C
���������������������ԣ�������ͭ��I����S2������ˮ���ء���FeCl3��Һ���������ӵ�ˮ��ƽ��Fe3����3H2O Fe(OH)3��3H�������ȴٽ�ˮ�⣬���Խ�FeCl3��Һ�������ɵõ������������������յõ���������������ˮ�������ԣ�̼������ˮ���Լ��ԣ�������ٽ���������ˮ�������ԣ���˼��������������ˮ�⡣����C��
Fe(OH)3��3H�������ȴٽ�ˮ�⣬���Խ�FeCl3��Һ�������ɵõ������������������յõ���������������ˮ�������ԣ�̼������ˮ���Լ��ԣ�������ٽ���������ˮ�������ԣ���˼��������������ˮ�⡣����C��

��ϰ��ϵ�д�
�����Ŀ
��1����4�֣������й�ʵ�����������˵������ȷ����
| A����pH��ֽ������ˮʪ�����ij��Һ��pH |
| B����ͭƬ����Ƭ������һ�����ϡ�����У�ͭƬ����������� |
| C���ζ���ϴ��������ˮ��ϴ����ע���Һ�����к͵ζ�ʵ�� |
| D�����������Һ���������������ͨ��һ����HCl����ʱ���������Һ�ɻָ�����ԭ��һ�� |
F��ʵ����������FeCl3��Һʱ���Ƚ�FeCl3����һ������Ũ�����У��ټ�����ˮϡ��������Ũ��
��2����6�֣�ijͬѧ�ڰ�����ʦ����ʵ���ҵĻ�ѧ�Լ�ʱ������һʢ����ɫ��Һ���Լ�ƿ����ǩ����(����ͼ)��������������յ�֪ʶ���Ը��Լ�������ʲô���ʵ���Һ�������룬�����ʵ�������֤��

�ٲ��룺�����Լ�������_____________________��
�ڼ�����֤��ʵ�鷽���� ��