��Ŀ����
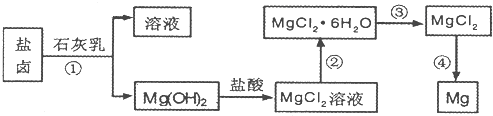
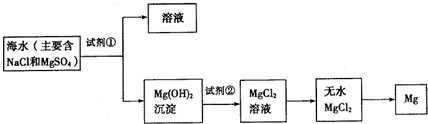
Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ���ͼ��ij�����Ӻ�ˮ����ȡþ����Ҫ���裮ѧ�����������չ�������ۣ�
ѧ�����������������������⣺
��һ���ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵĸ�����������ѧ������Լ��Ĺ۵㣮
ѧ���Ĺ۵㣺ֱ������ˮ�м����������
ѧ���ҵĹ۵㣺���¼���������ˮ���ټ����������
ѧ�����Ĺ۵㣺����ɹ�κ�Ŀ�±ˮ���ټ����������
ͨ�������Ƚ�����Ϊѧ�� �Ĺ۵���ȷ����ѧ����ţ����������ɣ� ��
�������ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵķ��룿
��1��Ϊ��ʹþ���ӳ�����������������õ��صı��ǣ���Ҫ�ɷ�Ϊ̼��ƣ���Դ������������Լ����� ���ѧʽ����
��2�������Լ��ٺ��ܹ�����õ�Mg��OH��2�����ķ����� ��������ĸ��
A������ B������ C����ȡ D����Һ
��3������������Լ����� ���ѧʽ����
��4��д������ˮMgCl2��ȡ����þ�Ļ�ѧ����ʽ ��

ѧ�����������������������⣺
��һ���ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵĸ�����������ѧ������Լ��Ĺ۵㣮
ѧ���Ĺ۵㣺ֱ������ˮ�м����������
ѧ���ҵĹ۵㣺���¼���������ˮ���ټ����������
ѧ�����Ĺ۵㣺����ɹ�κ�Ŀ�±ˮ���ټ����������
ͨ�������Ƚ�����Ϊѧ�� �Ĺ۵���ȷ����ѧ����ţ����������ɣ� ��
�������ں�ˮ��þ�Ĺ��������ʵ�ֶ�þ���ӵķ��룿
��1��Ϊ��ʹþ���ӳ�����������������õ��صı��ǣ���Ҫ�ɷ�Ϊ̼��ƣ���Դ������������Լ����� ���ѧʽ����
��2�������Լ��ٺ��ܹ�����õ�Mg��OH��2�����ķ����� ��������ĸ��
A������ B������ C����ȡ D����Һ
��3������������Լ����� ���ѧʽ����
��4��д������ˮMgCl2��ȡ����þ�Ļ�ѧ����ʽ ��
��һ��ѧ�����������۵���ȣ���ˮ��þ����Ũ��С��ʹ�õij����������ϴ��Ҳ������ռ�������þ�����ѧ���Ĺ۵㲻��ȷ��ԭ���ǣ���ˮ��þ����Ũ��С��������������������þ���ӵij�����
ѧ�����������۵���ȣ�����������ˮ�����ĵ���Դ�࣬�ɱ�̫�ߣ����ѧ���ҵĹ۵㲻��ȷ��ԭ���ǣ���Դ���Ĵ�ˮ���ۺ����õͣ��ɱ��ߣ�
ѧ������ѧ������ȣ�����ɹ�κ�Ŀ�±����Լ��Դ���ɱ��ͣ���ѧ�������þ����Ũ�ȸߣ�������þԪ�صĸ��������ѧ�����Ĺ۵���ȷ����Ϊ��þ���Ӹ���Ũ�ȸߣ��ɱ��ͣ�
�ʴ�Ϊ������þ���Ӹ���Ũ�ȸߣ���Դ����С���ɱ��ͣ�
��������1����̼��Ƹ��·ֽ�õ������ƣ���������ˮ��Ӧ�����������ƣ��������������Ȼ�þ��Ӧ����������þ���ʴ�Ϊ��Ca��OH��2����CaO����
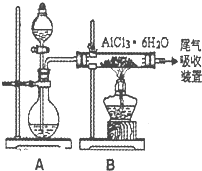
��2����Mg��OH��2������ˮ�����ù��˵ķ������з��룬��ѡ��B��
��3��Ҫʹ������þת��Ϊ�Ȼ�þ������Ҫ�������ᷴӦ�����Լ��������ᣬ�ʴ�Ϊ��HCl��
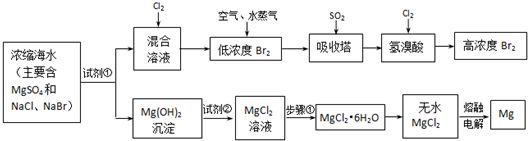
��3����ҵ��ȡþʱ���������״̬���Ȼ�þ��MgCl2
Mg+Cl2�����ʴ�Ϊ����MgCl2
Mg+Cl2����
ѧ�����������۵���ȣ�����������ˮ�����ĵ���Դ�࣬�ɱ�̫�ߣ����ѧ���ҵĹ۵㲻��ȷ��ԭ���ǣ���Դ���Ĵ�ˮ���ۺ����õͣ��ɱ��ߣ�
ѧ������ѧ������ȣ�����ɹ�κ�Ŀ�±����Լ��Դ���ɱ��ͣ���ѧ�������þ����Ũ�ȸߣ�������þԪ�صĸ��������ѧ�����Ĺ۵���ȷ����Ϊ��þ���Ӹ���Ũ�ȸߣ��ɱ��ͣ�
�ʴ�Ϊ������þ���Ӹ���Ũ�ȸߣ���Դ����С���ɱ��ͣ�
��������1����̼��Ƹ��·ֽ�õ������ƣ���������ˮ��Ӧ�����������ƣ��������������Ȼ�þ��Ӧ����������þ���ʴ�Ϊ��Ca��OH��2����CaO����
��2����Mg��OH��2������ˮ�����ù��˵ķ������з��룬��ѡ��B��
��3��Ҫʹ������þת��Ϊ�Ȼ�þ������Ҫ�������ᷴӦ�����Լ��������ᣬ�ʴ�Ϊ��HCl��
��3����ҵ��ȡþʱ���������״̬���Ȼ�þ��MgCl2
| ||
| ||

��ϰ��ϵ�д�
 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
�����Ŀ

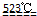 MgO+2 HCl��+5H2O��
MgO+2 HCl��+5H2O��