��Ŀ����
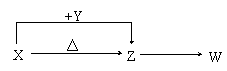
��W�Ļ�ѧʽ��____________________��
��X��Y����Һ�з�Ӧ�����ӷ���ʽ��________________________��
�Ǣٽ�4.48 L(������Ϊ��״��)Wͨ��100 mL 3 mol/L��Y��ˮ��Һ����Һ�е�������_________��
����Ȼ���д���X��Z��H2O��һ�������ᾧ���ɵĹ��塣ȡһ�����ù�������ˮ���100 mL��Һ�������Һ�н��������ӵ�Ũ��Ϊ0.5 mol/L����ȡ��ͬ�����Ĺ�����������أ�ʣ����������Ϊ____________ g��
��ѡ��X�����е�����Ԫ���е����ֻ�������ɵĻ������������ͼװ��(�г̶ֹ�װ������ȥ)����ʵ�飬װ�â��в�����ɫ������װ�â��п��ռ���һ����ɫ��ȼ�����塣
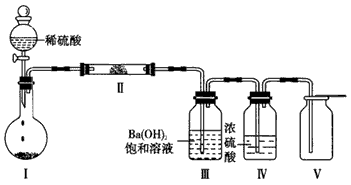
����X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ����������װ�â������壬�û�����Ļ�ѧʽ��____________����������װ����__________(����ͼѡ���Ҫװ�ã���д���)��
��HCO3-+ OH-== CO32- + H2O
�Ǣ�Na2CO3��NaHCO3����2.65
�Ȣ�Na2CO3 + H2SO4 == Na2SO4 + CO2��+ H2O��Na2O2����H2O2��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�X��Y��Z��W��Ԫ�����ڱ���ԭ������������������ֶ�����Ԫ�أ��������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
X |
ơ�ƺ������г�����X������������ƿ��ʱ�����������������ݳ� |
|
Y |
Y2�ǿ����к�����ߵ����嵥�� |
|
Z |
Z�Ļ�̬ԭ�����������Ų�ʽΪ2s22p4 |
|
W |
W��һ�ֺ���������Ϊ27��������Ϊ14 |
��1��Wλ��Ԫ�����ڱ��� ���ڵ� �壻Z��W��������Ӱ뾶��С��ϵΪ
�������ӷ��ű�ʾ��
��2��һ��X�γɵĵ��ʣ�����Ȼ��Ӳ���������ʣ����ۻ����ֵ��ʣ�����˷���������������� ��Y�ĵ縺�Ա�Z �����С����
��3��W�ĵ�����NaOH��Һ��Ӧ�����ӷ���ʽΪ �� W�ڸ��������¿ɻ�ԭ���۵�Ľ����������ƵĽ������ʣ�д��W��ԭCr2O3��Ӧ�Ļ�ѧ����ʽ�� ��
��4��úȼ�ղ�������������Y����������������صĻ������⣬��ˣ�����XH4����ԭ��������Ⱦ����֪��
XH4(g)+2 YO2(g)== Y2(g)+ XO2(g)+2H2O (g) ��H1= -867kJ/mol
2 YO2(g)  Y2O4(g)
��H2=-56.9kJ/mol
Y2O4(g)
��H2=-56.9kJ/mol
��11�֣�X��Y��Z��W��Ԫ�����ڱ�ǰ�������е����ֳ���Ԫ�أ��������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
X |
X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
|
Y |
���³�ѹ�£�Y�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
|
Z |
Z��Yͬ����,Z�ĵ縺�Դ���Y |
|
W |
W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��1��Y��Ԫ�ط���������������
��2��XY2��һ�ֳ��õ��ܼ���XY2�ķ����д������������Ҽ�������ԭ���ӻ�������� ����H�DY��H�DZ���ֹ��ۼ��У����ļ��Խ�ǿ�������������������ϳ�����������������
��3��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��������������������������
��4��������XO��YO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����Y���˷�Ӧ�Ļ�ѧ����ʽ������������������������������������������
(5)�����һ��ʵ�鷽�����Ƚ�Y��Z���������Ե�ǿ����
_____________________________________________________________________________________________________________________________________��