��Ŀ����
һ���¶Ⱥ�ѹǿ�£�30Lij����̬�������к���6.02×1023�����ӣ���Щ������1.204×1024��ԭ����ɣ������й�˵���в���ȷ���ǣ� ��A����״���¸ô�������Ϊ��̬�������Լ��22.4 L
B����������ÿ�����Ӻ���2��ԭ��
C����O2�ڸ�������Ϊ��̬����1 mol O2�ڸ������µ����ҲΪ30 L
D�����¶Ⱥ�ѹǿ�����DZ�״��
���𰸡�������A���ڱ�״����6.02×1023�����ӣ������ʵ���Ϊ1mol�����Ϊ22.4L��
B������n=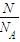 �������ʵ������������ʵ���Ϊ1mol��ԭ�����ʵ���Ϊ2mol��˵����˫ԭ�ӷ��ӣ�
�������ʵ������������ʵ���Ϊ1mol��ԭ�����ʵ���Ϊ2mol��˵����˫ԭ�ӷ��ӣ�
C��һ���¶�ѹǿ�£����ʵ�����ͬ�����������ͬ�������
D��1mol�����ڱ�״�����Ϊ22.4L��
����⣺һ���¶Ⱥ�ѹǿ�£�30Lij����̬�������к���6.02×1023���������ʵ���Ϊ1mol����Щ������1.204×1024��ԭ����ɼ�2molԭ�ӹ��ɣ�
A����״���¸ô�������Ϊ��̬�����ʵ���Ϊ1mol�������Լ��22.4 L����A��ȷ��
B�����ݼ���1mol�����к���2molԭ�ӣ���������ÿ�����Ӻ���2��ԭ�ӣ���B��ȷ��
C��ͬ�¡�ͬѹ��ͬ���ʵ��������������ͬ��������O2�ڸ�������Ϊ��̬����1 mol O2�ڸ������µ����ҲΪ30 L����C��ȷ��
D�����1mol��������Ϊ30ml�����Ը��¶Ⱥ�ѹǿ�������DZ�״�����ڱ�״��1mol�������ԼΪ22.4L����D����
��ѡD��
���������⿼�������ʵ����ļ���Ӧ�ã�����٤�����ɵķ����жϣ�����Ħ�����������Ӧ�ã���Ŀ�ϼ�
B������n=
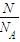 �������ʵ������������ʵ���Ϊ1mol��ԭ�����ʵ���Ϊ2mol��˵����˫ԭ�ӷ��ӣ�
�������ʵ������������ʵ���Ϊ1mol��ԭ�����ʵ���Ϊ2mol��˵����˫ԭ�ӷ��ӣ�C��һ���¶�ѹǿ�£����ʵ�����ͬ�����������ͬ�������
D��1mol�����ڱ�״�����Ϊ22.4L��
����⣺һ���¶Ⱥ�ѹǿ�£�30Lij����̬�������к���6.02×1023���������ʵ���Ϊ1mol����Щ������1.204×1024��ԭ����ɼ�2molԭ�ӹ��ɣ�
A����״���¸ô�������Ϊ��̬�����ʵ���Ϊ1mol�������Լ��22.4 L����A��ȷ��
B�����ݼ���1mol�����к���2molԭ�ӣ���������ÿ�����Ӻ���2��ԭ�ӣ���B��ȷ��
C��ͬ�¡�ͬѹ��ͬ���ʵ��������������ͬ��������O2�ڸ�������Ϊ��̬����1 mol O2�ڸ������µ����ҲΪ30 L����C��ȷ��
D�����1mol��������Ϊ30ml�����Ը��¶Ⱥ�ѹǿ�������DZ�״�����ڱ�״��1mol�������ԼΪ22.4L����D����
��ѡD��
���������⿼�������ʵ����ļ���Ӧ�ã�����٤�����ɵķ����жϣ�����Ħ�����������Ӧ�ã���Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
���й���˵����ȷ���ǣ�������
| A��HCl��NaOH��Ӧ���к��ȡ�H=-57.3kJ/mol����H2SO4��Ba��OH��2��Ӧ���к��ȡ�H=2����-57.3��kJ/mol | B����֪C2H5OH��l�� ��ȼ������1366.8KJ/mol����C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��g����Ӧ�ġ�H=-1366.8kJ/mol | C��һ��������2SO2��g��+O2��g��?2SO3��g����H1��2SO2��g��+O2��g��?2SO3��l����H2���H1����H2 | D����һ���¶Ⱥ�ѹǿ�£���0.5mol N2��1.5mol H2�����ܱ������г�ַ�Ӧ����NH3��g�����ų�����19.3kJ�������Ȼ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-38.6kJ/mol |
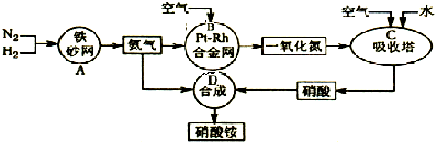
 ��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺