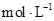��Ŀ����

����ȡ50mL 0��25 mol/L���ᵹ��С�ձ��У������¶ȣ�
����ȡ50mL 0��55mol/L NaOH��Һ�������¶ȣ�
�۽�NaOH��Һ����С�ձ���,��Ͼ��Ⱥ�������Һ�¶ȡ�
��ش�
��1��NaOH��Һ�Թ�����ԭ�� ____________��
��2������NaOH��Һ����ȷ������_____________ ������ĸ����
A���ز�������������
B��һ��Ѹ�ټ���
C���������
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������__________ ��
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18 J����g���棩-1��
�����ʵ������д���÷�Ӧ���Ȼ�ѧ����ʽ_____________ ��

��2��B
��3���û��β������������
��4��H2SO4��aq��+2NaOH��aq��== Na2SO4��aq��+2H2O��l������H=-113��6kJ��mol-1
��5�����ڣ� Ũ��������ˮ�ų�����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�(12��)������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������Ͳ��ȡ50 mL 0.25 mol/L���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol/L NaOH��Һ��������һ�¶ȼƲ�����¶ȣ�

�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ�������Һ���
�¶ȡ��ش��������⣺
(1)д��ϡ�����ϡ����������Һ��Ӧ��ʾ�к��ȵ��Ȼ�ѧ����ʽ(�к�����ֵΪ
57.3 kJ/mol)��_______________________________________________��
(2)����NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز������������롡 B���������������� C��һ��Ѹ�ٵ���
(3)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β���������ؽ���
(4)ʵ���������±���
������д�±��еĿհף�
|
�¶� ʵ������� |
��ʼ�¶�t1�� |
��ֹ�¶�t2/�� |
�¶Ȳ�ƽ��ֵ (t2��t1)/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
26.2 |
26.0 |
26.1 |
29.5 |
|
|
2 |
27.0 |
27.4 |
27.2 |
32.3 |
|
|
3 |
25.9 |
25.9 |
25.9 |
29.2 |
|
|
4 |
26.4 |
26.2 |
26.3 |
29.8 |
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к��Ȧ�H��__________ ( ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)__________��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���


 ��Һ��
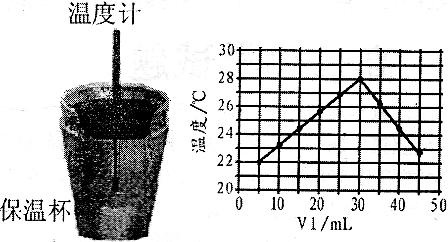
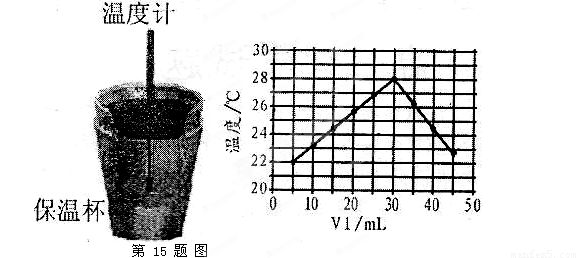
��Һ�� δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ��� )��
)�� (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
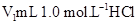 ��Һ��
��Һ��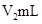 δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���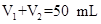 )��
)�� (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��