��Ŀ����
ij�����7.8g�����������ᷴӦ�ų�8.96L��״���µ����壬�˻�����������ۺ����������ֽ�����϶��ɣ������۽�����ʽ���������۽���ʽ���� �����һ����������ԭ�Ӹ�����Ϊ1��2��
�����һ����������ԭ�Ӹ�����Ϊ1��2��
(1)д�������ֽ�����ʽ����Ԫ�ط��ţ�
(2)��֪���۽���ԭ�Ӻ��ڵ�����������������ȣ����۽���ԭ�Ӻ��ڵ�����������������1�������Ʋ������ֽ���Ԫ�������ڱ��е�λ�ã�
������
|
����(1)���۽�����ʽ��Ϊ24��Mg�����۽�����ʽ��Ϊ27��Al ����(2)Mgλ�ڵ������ڢ�A�壻Alλ�ڵ������ڢ�A�� |

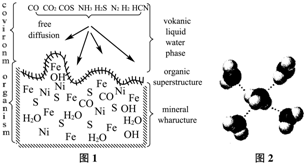
A. ������Һ�о�(ͼ1)���ڵ�����е�ǰ�ء�������Һ������̴ѡ�����Χ������FeS����ͭ��п��ȿ��
(1) Ni2���ĺ�������Ų�ʽ��____________________��
(2) �����±���ͭ�ĵ�һ������(I1)С��п�ĵ�һ�����ܣ���ͭ�ĵڶ�������(I2)ȴ����п�ĵڶ������ܣ�����Ҫԭ���ǡ���
����
| ������/kJ��mol��1 | I1 | I2 |
| ͭ | 746 | 1958 |
| п | 906 | 1733 |
(3) ����˵����ȷ����________��
A. �縺�ԣ�N��O��S��C����������������B. CO2��COS(����)��Ϊ�ȵ�����
C. NH3�����е�ԭ�Ӳ���sp3�ӻ� D. CO��H2S��HCN���Ǽ��Է���
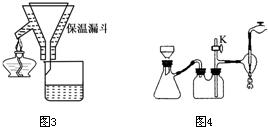
(4) ��������Һ���д�������һ�������ӣ��ṹ��ͼ2�����Ļ�ѧʽΪ________________��
(5) FeS��NaCl��Ϊ���Ӿ��壬�������ƣ�ǰ���۵�Ϊ985�棬����801�棬��ԭ����____________________________________________����FeS�����У���Fe2����������������S2��Χ�ɵĶ�����Ŀռ乹��Ϊ________________��
B. �Ʊ�KNO3�����ʵ�������ýᾧ���ؽᾧ����KNO3��NaCl�Ļ������з��롣������ij��ѧ��ȤС��Ļ��¼��
|
| NaNO3 | KNO3 | NaCl | KCl |
| 10�� | 80.5 | 20.9 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |
�������ϣ������в�ã��������ڲ�ͬ�¶��µ��ܽ��(S/g)���±���
ʵ�鷽����
��. �ܽ⣺��ȡ29.8g KCl��34.0g NaNO3����250mL�ձ��У��ټ���70.0g����ˮ�����Ȳ����裬ʹ����ȫ���ܽ⡣
��. �����ᾧ���������Ⱥͽ��裬����Һ����Ũ������100��ʱ������50.0g ˮ��ά�ָ��¶ȣ��ڱ���©��(��ͼ��ʾ)�г��ȹ��������ľ��塣�þ���m1g��
��. ��ȴ�ᾧ������Һ��ȴ������(ʵ��ʱ����Ϊ10��)���м�ѹ���ˡ���KNO3�ֲ�Ʒm2g��
��. �ؽᾧ�����ֲ�Ʒȫ������ˮ���Ƴ�100��ı�����Һ����ȴ�����º���ˡ���KNO3��Ʒ��
�ٶ����� �����ʱ��Ӱ����Ե��ܽ�ȣ��� ���ֹ��˲��������У��ܼ�����ĺ��Բ��ơ��Իش��й����⣺
(1) �������г��ȹ��˵�Ŀ���ǡ���
(2) ���������гн���Һ���ձ��в���������ˮ�����������ڲ������пɵôֲ�Ʒ������m2��______g�����л���NaCl______g��Ϊ��ֹNaCl���룬�ڲ������гн���Һ���ձ�������Ӧ��������ˮ______g��
(3) �������в��ü�ѹ���ˣ����ŵ���______________________________________����С��ͬѧ���õ�װ������ͼ��ʾ����д����װ������Ҫ�õ��IJ������������ƣ�________________����ʵ������з��ֵ�������Ӧ��ȡ�Ĵ�ʩ��______________________________________��
��18�֣�
����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�ij������ȤС�齫����Ũ����ֶ�μӵ�ͭ����ϡ ����Ļ�����У�����ʹ֮��Ӧ��ȫ��װ������29ͼI��ͼ����ʾ����ͨ���������ᾧ�õ�����ͭ���壬��ͬʱ�ⶨ����ͭ�����нᾧˮ�ĺ�����
��1���������ͼIװ�õ������� ��
��2��ͼI�з�Һ©����װ��Һ���� ��
��3��ͼ����ͼ��ĸĽ�װ�ã���ͼI��ȣ�ͼ��װ�õ������ŵ��� ��
�� �����ɲ���������
��4��������ȤС��ͬѧ�ڲⶨ���� �����нᾧˮ�ĺ���ʱ������й��������±���
�����нᾧˮ�ĺ���ʱ������й��������±���
| ����ǰ���� | ���Ⱥ����� | |
| m1�������� | m2������+���壩 | m3������+��ˮCuSO4�� |
| 5.4g | 7.9g | 6.8g |
��Ϊ��ɲⶨ����29ͼ���л�ȱ�ٵ����������� ��
���ж���Ʒ�Ѻ��صķ����� ��
�۲ⶨ���õ���![]() �нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�нᾧˮ������ʵ���У������������ٽ��� �Ρ�
�ܿ�����ȤС��IJⶨ���xֵ���� ��ȣ� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
��ȣ� ���ƫ�ߡ�����ƫ�͡��������䡱�������ܵ�ԭ���� ��������ĸ��ţ�
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯
 �������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�ʹ������������������ⶼ������A�����֣�
�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݣ���ѡ������һ�⣬������Ӧ�ʹ������������������ⶼ������A�����֣�