��Ŀ����
�������õ���ͨ���Ũ��NaOH��H2O2�Ļ��Һ�У��ڵ��ܿ�����Һ�ĽӴ�������˸�ĺ����֣�������Ϊͨ������Һ�в�����ClO����H2O2��ԭ���������ҷ�Ӧ�����������ϸߵ������ӣ�������ת��Ϊ��ͨ�����ӣ�������������Ժ��ų���
���д�ʵ�飬���õ�������������ͼ��
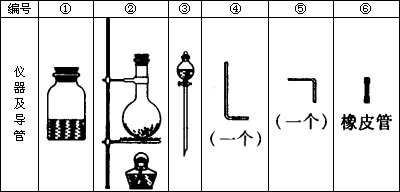
����Ҫ����д���пհף�
(1)��װ����������ʱ��Ӧѡ�õ�����������(��дͼ���)��________��
(2)ʵ������У���������������ҵ�˳�����������ĸ����������ܵı��������________��
(3)�����ٵ���Ƥ����Ӧ��________���ף�ԭ����________��
(4)ʵ��ʱ���������г��۲쵽������________����
(5)ʵ������Լ10����H2O2��Һ100��������������30��(�ܶȽ���Ϊ1 g/cm3)H2O2�����ƣ���������Ʒ����ǣ�________________________________��
(6)ʵ��ʱ��������ClO����H2O2��Ӧ�����ӷ���ʽ�ǣ�________��
������
|
����[��](1)�ۢڢ� ����(2)�ڢݢޢܢٻ�ۢڢݢޢܢ� ����(3)2��ʹƿ����ѹǿ��� ����(4)ð���� ����(5)��H2O2���x mL��x��1��30����100��10�� ����x��33.3 mL ��������Ͳ��ȡ33 mL��30����H2O2��Һ�����ձ��У��ټ�67 mLˮ��������� ����(6) ����[����]��װ��������װ�ã�Ӧѡ��ڡ��ۺ�һ֧���ܣ������ܢܡ���ÿ�ֽ���1��������ƿ�е���Cl2Ӧѡ�ݣ��Ѣ����������ͨ��Cl2�ã� ��������������������Ӧ�Ӣڿ�ʼ����Ϊֹ���м������Ǣݡ��ޡ��ܣ� �������в�����Cl2������ͨ��ٵĻ��Һ�з�����Ӧ����Ҫ������ˮ���ܽ��Բ��õ�O2�����Ԣٵ���Ƥ���ϼ�Ҫ�����嵼��Ŀף���Ҫ�ж���Cl2�������ɵ�O2�����Ŀף� ����ʵ������Լ10��H2O2��Һ100 mL�� ����ClO����H2O2�ķ�Ӧ��������ԭ��Ӧ��������ClO����H2O2��ԭ������O2����ˣ�ClO������������H2O2�ǻ�ԭ��������������O2����ԭ������ClO������NaOH��Һ��ClO���Ļ�ԭ����ֻ����Cl���� |





