��Ŀ����
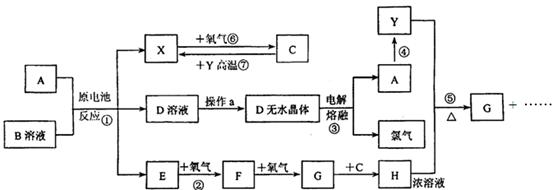
��֪AΪ����������X��YΪ�����ǽ�����X��E��F��G������Ϊ���壬CΪҺ�壬B��һ���Σ����ȼ��ֽ⣬�ڹ�ũҵ��������;�Ϲ㣨�类����ijЩ��صĵ���ʣ�������A��ʯī���缫��B��Ũ��Һ������ʣ�����ԭ��ء��й�����֮���ת����ϵ����ͼ����ע�⣺������Щ��Ӧ�����������������ﱻ��ȥ��

����д���пհ�
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ��_________________��
��2����D��Һ�Ʊ�D����ˮ����IJ���aΪ��_______________��
��3����Ӧ�ڵĻ�ѧ����ʽΪ��_______________________��
��4����Ӧ�ݵĻ�ѧ����ʽΪ��_______________________��
��5��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ��_______________________��
��1����Ӧ��ΪA��ij��������ȼ�գ����ɵ���Y��A��������䷴Ӧ����ʽΪ��_________________��
��2����D��Һ�Ʊ�D����ˮ����IJ���aΪ��_______________��
��3����Ӧ�ڵĻ�ѧ����ʽΪ��_______________________��
��4����Ӧ�ݵĻ�ѧ����ʽΪ��_______________________��
��5��ԭ��ط�Ӧ���������ĵ缫��ӦʽΪ��_______________________��
��1��2Mg+CO2==2MgO+C
��2����D��Һ��HCl����������
��3����4NH3+5O2 4NO+6H2O
4NO+6H2O
��4����C+4HNO3(Ũ) CO2+4NO2��+2H2O
CO2+4NO2��+2H2O
��5��2NH4++2e-==2NH3��+H2��
��2����D��Һ��HCl����������
��3����4NH3+5O2
 4NO+6H2O
4NO+6H2O��4����C+4HNO3(Ũ)
 CO2+4NO2��+2H2O
CO2+4NO2��+2H2O��5��2NH4++2e-==2NH3��+H2��

��ϰ��ϵ�д�
�����Ŀ