��Ŀ����
0.2molij��A����������ȫȼ�պ�����CO2��H2O��1.2mol���Իش���1����A�ķ���ʽΪ ��
��2����ȡһ�����ĸ���A��ȫȼ�պ�����CO2��H2O��3mol������ g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ����� L��
��3������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ ��
��4������A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4��������A�����еĽṹ��ʽΪ ������д1����
��5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ���� ��ͬ���칹�壮
���𰸡���������1����0.2molij��A����������ȫȼ�պ�����CO2��H2O��1.2mol������ԭ���غ�ȷ����A������C��Hԭ����Ŀ����֪��A����ʽΪC6H12��
��2������Cԭ���غ����μӷ�Ӧ����A�����ʵ������ٸ���m=nM����μӷ�Ӧ��A��������һ�����ʵ�������������Ϊ���ģ�x+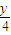 �������ٸ���V=nVm���������������
�������ٸ���V=nVm���������������
��3����A����ʽΪC6H12����A����ʹ��ˮ��ɫ�����������ͼ�����һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�AΪ�����飻
��4����A��ʹ��ˮ��ɫ������1��C=C˫�����ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4�������ʼӳɲ���Ľṹ��ʽΪ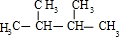 ��
��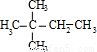 ������Cԭ���ϸ�ȥ��1��Hԭ�ӣ���ԭC=C˫����
������Cԭ���ϸ�ȥ��1��Hԭ�ӣ���ԭC=C˫����
��5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ�����ʽΪC5H10������1��C=C˫�����ݴ���д����������ͬ���칹���жϣ�
����⣺��1����0.2molij��A����������ȫȼ�պ�����CO2��H2O��1.2mol������ԭ���غ�ȷ��A������Cԭ����ĿΪ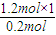 =6��Hԭ����ĿΪ
=6��Hԭ����ĿΪ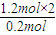 =12������A����ʽΪC6H12���ʴ�Ϊ��C6H12��
=12������A����ʽΪC6H12���ʴ�Ϊ��C6H12��
��2������Cԭ���غ��֪�μӷ�Ӧ����A�����ʵ���Ϊ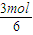 =0.5mol���ʲμӷ�Ӧ��A������Ϊ0.5mol×84g/mol=42g�����������������ʵ���Ϊ0.5mol×��6+
=0.5mol���ʲμӷ�Ӧ��A������Ϊ0.5mol×84g/mol=42g�����������������ʵ���Ϊ0.5mol×��6+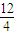 ��=4.5mol����״�������������Ϊ4.5mol×22.4L/mol=100.8L��
��=4.5mol����״�������������Ϊ4.5mol×22.4L/mol=100.8L��
�ʴ�Ϊ��42g��100.8��
��3����A����ʽΪC6H12����A����ʹ��ˮ��ɫ�����������ͼ�����һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�AΪ�����飬�ṹ��ʽΪ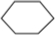 ���ʴ�Ϊ��
���ʴ�Ϊ��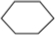 ��
��
��4����A��ʹ��ˮ��ɫ������1��C=C˫�����ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4�������ʼӳɲ���Ľṹ��ʽΪ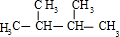 ��
��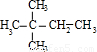 �����ӳɲ���Ϊ
�����ӳɲ���Ϊ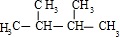 ����Ӧ��A�ĽṹΪ��CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2�����ӳɲ���Ϊ
����Ӧ��A�ĽṹΪ��CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2�����ӳɲ���Ϊ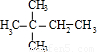 ����Ӧ��A�ĽṹΪ��CH3��3CCH=CH2��
����Ӧ��A�ĽṹΪ��CH3��3CCH=CH2��
�ʴ�Ϊ����CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2��CH3��3CCH=CH2��
��5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ�����ʽΪC5H10������1��C=C˫��������������ͬ���칹��Ϊ��CH3CH2CH2CH=CH2��CH3CH2CH=CHCH3��CH2=CH��CH3��CH2CH3����CH3��2C=CHCH3����CH3��2CHCH=CH2���ʴ�Ϊ��5��
���������⿼���л�����ƶϡ�ϩ�������ʡ�ͬ���칹�塢��ѧ����ȣ��Ѷ��еȣ�ע�⣨4���и��ݼӳɷ�Ӧԭ�������û�ԭC=C˫����������д��
��2������Cԭ���غ����μӷ�Ӧ����A�����ʵ������ٸ���m=nM����μӷ�Ӧ��A��������һ�����ʵ�������������Ϊ���ģ�x+
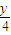 �������ٸ���V=nVm���������������
�������ٸ���V=nVm�����������������3����A����ʽΪC6H12����A����ʹ��ˮ��ɫ�����������ͼ�����һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�AΪ�����飻
��4����A��ʹ��ˮ��ɫ������1��C=C˫�����ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4�������ʼӳɲ���Ľṹ��ʽΪ
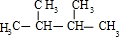 ��
��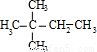 ������Cԭ���ϸ�ȥ��1��Hԭ�ӣ���ԭC=C˫����
������Cԭ���ϸ�ȥ��1��Hԭ�ӣ���ԭC=C˫������5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ�����ʽΪC5H10������1��C=C˫�����ݴ���д����������ͬ���칹���жϣ�
����⣺��1����0.2molij��A����������ȫȼ�պ�����CO2��H2O��1.2mol������ԭ���غ�ȷ��A������Cԭ����ĿΪ
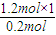 =6��Hԭ����ĿΪ
=6��Hԭ����ĿΪ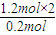 =12������A����ʽΪC6H12���ʴ�Ϊ��C6H12��
=12������A����ʽΪC6H12���ʴ�Ϊ��C6H12����2������Cԭ���غ��֪�μӷ�Ӧ����A�����ʵ���Ϊ
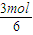 =0.5mol���ʲμӷ�Ӧ��A������Ϊ0.5mol×84g/mol=42g�����������������ʵ���Ϊ0.5mol×��6+
=0.5mol���ʲμӷ�Ӧ��A������Ϊ0.5mol×84g/mol=42g�����������������ʵ���Ϊ0.5mol×��6+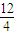 ��=4.5mol����״�������������Ϊ4.5mol×22.4L/mol=100.8L��
��=4.5mol����״�������������Ϊ4.5mol×22.4L/mol=100.8L���ʴ�Ϊ��42g��100.8��
��3����A����ʽΪC6H12����A����ʹ��ˮ��ɫ�����������ͼ�����һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�AΪ�����飬�ṹ��ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4����A��ʹ��ˮ��ɫ������1��C=C˫�����ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4�������ʼӳɲ���Ľṹ��ʽΪ
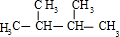 ��
��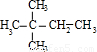 �����ӳɲ���Ϊ
�����ӳɲ���Ϊ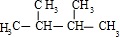 ����Ӧ��A�ĽṹΪ��CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2�����ӳɲ���Ϊ
����Ӧ��A�ĽṹΪ��CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2�����ӳɲ���Ϊ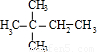 ����Ӧ��A�ĽṹΪ��CH3��3CCH=CH2��
����Ӧ��A�ĽṹΪ��CH3��3CCH=CH2���ʴ�Ϊ����CH3��2CHC��CH3��=CH2��CH3��2C=C��CH3��2��CH3��3CCH=CH2��
��5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ�����ʽΪC5H10������1��C=C˫��������������ͬ���칹��Ϊ��CH3CH2CH2CH=CH2��CH3CH2CH=CHCH3��CH2=CH��CH3��CH2CH3����CH3��2C=CHCH3����CH3��2CHCH=CH2���ʴ�Ϊ��5��
���������⿼���л�����ƶϡ�ϩ�������ʡ�ͬ���칹�塢��ѧ����ȣ��Ѷ��еȣ�ע�⣨4���и��ݼӳɷ�Ӧԭ�������û�ԭC=C˫����������д��

��ϰ��ϵ�д�
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
�����Ŀ