��Ŀ����
��16�֣��״������͵���������ȼ�ϡ���ҵ�Ͽ�ͨ��H2��CO�����Ʊ��״����÷�Ӧ���Ȼ�ѧ����ʽΪ��2H2(g)��CO(g) CH3OH(g)
CH3OH(g) 
��1����֪��



1 mol�״�������ȫȼ������CO ��ˮ�������Ȼ�ѧ����ʽΪ ��
��ˮ�������Ȼ�ѧ����ʽΪ ��
��2�����д�ʩ�����������2H2(g)��CO(g) CH3OH(g)��Ӧ���ʵ��� ��˫ѡ)��
CH3OH(g)��Ӧ���ʵ��� ��˫ѡ)��
| A�������CH3OH | B�������¶� | C����Сѹǿ | D��������ʵĴ��� |

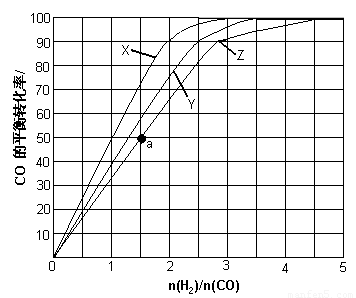
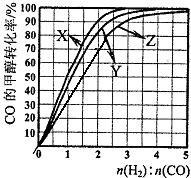
������X��Ӧ���¶��� ��
�ڴ�ͼ�п��Եó��Ľ����� ����дһ������
��4��������Ӧ���ݻ��м���1.5molH2��1.0molCO��������Z��Ӧ�¶��·�Ӧ��ƽ�⡣��������ͼ��a���Ӧ��COƽ��ת���ʣ�����2H2(g)��CO(g)
 CH3OH(g)��ƽ�ⳣ������д��������̣�
CH3OH(g)��ƽ�ⳣ������д��������̣�
(1) 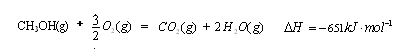 (3��)
(3��)
��2��BD ��4�֣� ��3����230�棨3�֣�
�������������䣬CO��ƽ��ת��������H2��CO����ʼ��ɱ���������������������䣬CO��ƽ��ת�������¶����߶����͡��� ��2�֣�
��4��a��H2��CO����ʼ��ɱ�Ϊ1.5��CO��ƽ��ת����Ϊ50������1�֣�
2H2(g)��CO(g) CH3OH(g)
CH3OH(g)
��ʼ���ʵ���(mol) 1.5 1 0
ת�����ʵ���(mol�� 1 0.5 0.5
ƽ�����ʵ���(mol) 0.5 0.5 0.5
ƽ��Ũ��(mol/L) 0.5 0.5 0.5 ��1�֣�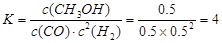 ��2�֣�
��2�֣�
����

��ϰ��ϵ�д�
�����Ŀ

 CH3OH(g)
CH3OH(g)
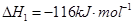
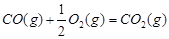
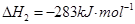
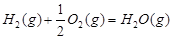
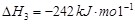
 ��ˮ�������Ȼ�ѧ����ʽΪ
��
��ˮ�������Ȼ�ѧ����ʽΪ
��