��Ŀ����
���Ṥҵ�д������·�Ӧ��2SO2(g)��O2(g) 2SO3(g) ��H<0
2SO3(g) ��H<0
��1��д���÷�Ӧ��ƽ�ⳣ������ʽ_______�������¶�ƽ�ⳣ��_______������䡱������������С������
��2���÷�Ӧ��ƽ�����ֻ����ѹǿ��ƽ�⽫��_______�����ƶ�����ֻ�����¶ȣ�ƽ����_______�����ƶ���
��3��ʵ��������Ҫ�ù����Ŀ�����ԭ����______________��������������Ŀ����______________�����ڸ����½��и÷�Ӧ��������______________��
 2SO3(g) ��H<0
2SO3(g) ��H<0 ��1��д���÷�Ӧ��ƽ�ⳣ������ʽ_______�������¶�ƽ�ⳣ��_______������䡱������������С������
��2���÷�Ӧ��ƽ�����ֻ����ѹǿ��ƽ�⽫��_______�����ƶ�����ֻ�����¶ȣ�ƽ����_______�����ƶ���
��3��ʵ��������Ҫ�ù����Ŀ�����ԭ����______________��������������Ŀ����______________�����ڸ����½��и÷�Ӧ��������______________��
��1��K=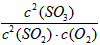 ����С
����С
��2������Ӧ���淴Ӧ
��3��������Դ�ḻ���ɱ��͵ķ�Ӧ��Ũ�ȣ����SO3�������ӿ췴Ӧ���ʣ��÷�ӦΪ���ȷ�Ӧ���¶�̫�߾��ܷ�Ӧ���ʿ죬��������
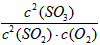 ����С
����С��2������Ӧ���淴Ӧ
��3��������Դ�ḻ���ɱ��͵ķ�Ӧ��Ũ�ȣ����SO3�������ӿ췴Ӧ���ʣ��÷�ӦΪ���ȷ�Ӧ���¶�̫�߾��ܷ�Ӧ���ʿ죬��������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ








