��Ŀ����
����Ŀ��������������![]() ��һ�ֻ�ɫ������ˮ�Ĺ��壬�����ֽ⣬������﮵�ء�Ϳ�ϡ���ɫ���Լ��й���ϵ�ԭ���ϡ�
��һ�ֻ�ɫ������ˮ�Ĺ��壬�����ֽ⣬������﮵�ء�Ϳ�ϡ���ɫ���Լ��й���ϵ�ԭ���ϡ�
I���Ʊ������������壺
![]() ��ȡ��������茶���
��ȡ��������茶���![]() ������
������![]() ������ƿ�У�����10������
������ƿ�У�����10������![]() ��Һ��
��Һ��![]() ����ˮ�����ܽ⣻
����ˮ�����ܽ⣻
![]() ����
����![]() ���Ͳ�����Һ�����Ƚ������У�ֹͣ���ȣ����ã�
���Ͳ�����Һ�����Ƚ������У�ֹͣ���ȣ����ã�
![]() ����ɫ����
����ɫ����![]() ������������ϴ�ӣ�����Լ
������������ϴ�ӣ�����Լ![]() ����ˮ���貢����
����ˮ���貢����![]() �����ã���ȥ�ϲ���Һ�����û�ɫ�����������塣
�����ã���ȥ�ϲ���Һ�����û�ɫ�����������塣

�ش��������⣺
��1������C��������_____________������B��������_____________
��2����������茶����������ܽ��ԭ���� _____________________
II��̽�����������Ĵ��ȣ�
![]() ȷ��ȡ
ȷ��ȡ![]() ��������������Ʒ
��������������Ʒ![]() ���в��������
����������![]() ������
������![]() ��Һ�У���
��Һ�У���![]() ˮԡ���ȣ���
ˮԡ���ȣ���![]() ����ƿ���
����ƿ���![]() ��Һ��
��Һ��
![]() ȡ������Һ
ȡ������Һ![]() ����
����![]() ����Һ
����Һ![]() Ũ��Ϊ
Ũ��Ϊ![]() �ζ����Σ�ƽ������
�ζ����Σ�ƽ������![]() ��
��
![]() ��������п�ۺ�
��������п�ۺ�![]() ��Һ����Ӧһ��ʱ���ȡ1����Һ���ڵ�ΰ��ϼ��飬��
��Һ����Ӧһ��ʱ���ȡ1����Һ���ڵ�ΰ��ϼ��飬��![]() ������
������
![]() ���˳�ȥп�ۣ���Һ�ռ�����һ����ƿ�У�����ֽ����������ϴ�ӣ�ϴ��Һ������Һ�У��ٲ���Լ
���˳�ȥп�ۣ���Һ�ռ�����һ����ƿ�У�����ֽ����������ϴ�ӣ�ϴ��Һ������Һ�У��ٲ���Լ![]() ��Һ��������
��Һ��������![]() ����Һ
����Һ![]() Ũ��Ϊ
Ũ��Ϊ![]() �ζ����յ㣬��ƽ�вⶨ���Σ�ƽ������
�ζ����յ㣬��ƽ�вⶨ���Σ�ƽ������![]() ��
��
�ش��������⣺
��3����![]() ����Һ�з���
����Һ�з���![]() ��������һ������ԭ��Ӧ��д�������ӷ���ʽ_______________________����
��������һ������ԭ��Ӧ��д�������ӷ���ʽ_______________________����![]() ����Һ�ζ����յ��������__________________��
����Һ�ζ����յ��������__________________��
��4����������п�۵�Ŀ����______________________________________������![]() �м�����������
�м�����������![]() ���Լ�������______________��Һ
���Լ�������______________��Һ
��5������![]() ������Ʒ�Ĵ���Ϊ______________
������Ʒ�Ĵ���Ϊ______________![]() �ú�c��
�ú�c��![]() ��
��![]() ��ʽ�ӱ�ʾ�����ػ���
��ʽ�ӱ�ʾ�����ػ���![]()
���𰸡���ѹ��Һ©�� �������� ����![]() ��ˮ��
��ˮ�� ![]() ���һ�α���Һ�������ƿ�е���Һ��ɫ�ɻ�ɫ��Ϊ���Ϻ�ɫ���Ұ�����ڲ���ɫ ��
���һ�α���Һ�������ƿ�е���Һ��ɫ�ɻ�ɫ��Ϊ���Ϻ�ɫ���Ұ�����ڲ���ɫ ��![]() ��ԭΪ
��ԭΪ![]() ���軯��
���軯�� ![]() ��
��
��������
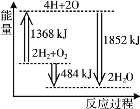
��1������װ��ͼ�ش�����C�����ƣ�����B�������ܣ�
��2������![]() ��ˮ�����ԭ��
��ˮ�����ԭ��
��3�������ܱ����Ը��������Һ�������������ӡ������������ȫ��Ӧ����ĸ������ʣ�ࡣ
��4��п�ۿ��Խ�![]() ��ԭΪ
��ԭΪ![]() ��
��![]() �����軯����Һ��Ѫ��ɫ��
�����軯����Һ��Ѫ��ɫ��
��5������![]() ������Ʒ���ȡ�
������Ʒ���ȡ�
![]() ����װ��ͼ��֪������C�������Ǻ�ѹ��Һ©��������B�������ܣ�������������������
����װ��ͼ��֪������C�������Ǻ�ѹ��Һ©��������B�������ܣ�������������������
![]() �����������
�����������![]() ��ˮ�⣬������������茶����������ܽ⣻
��ˮ�⣬������������茶����������ܽ⣻
![]() ��
��![]() ����Һ�л����в��ᣬ���ᱻ�������������������Ӧ�����ӷ���ʽΪ
����Һ�л����в��ᣬ���ᱻ�������������������Ӧ�����ӷ���ʽΪ![]() ����
����![]() ����Һ�ζ����յ�����������һ�α���Һ�������ƿ�е���Һ��ɫ�ɻ�ɫ��Ϊ���Ϻ�ɫ���Ұ�����ڲ���ɫ��
����Һ�ζ����յ�����������һ�α���Һ�������ƿ�е���Һ��ɫ�ɻ�ɫ��Ϊ���Ϻ�ɫ���Ұ�����ڲ���ɫ��
![]() ��������п�۵�Ŀ���ǽ�
��������п�۵�Ŀ���ǽ�![]() ��ԭΪ
��ԭΪ![]() ������
������![]() �����������
�м�����������![]() ���Լ����������軯����Һ�����������軯����Һʱ��Һ��ΪѪ��ɫ����
���Լ����������軯����Һ�����������軯����Һʱ��Һ��ΪѪ��ɫ����![]() ��
��
![]() ���ݷ�Ӧ
���ݷ�Ӧ![]() ��֪��
��֪��![]() ������Ʒ�Ĵ���Ϊ
������Ʒ�Ĵ���Ϊ ��
��

����Ŀ��25��ʱ��������ĵ���ƽ�ⳣ�����£�
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
�ش��������⣺
��1��һ������£����¶�����ʱ��Ka___(����������������С������������)��
��2�������������ӽ�����������ɴ�С��˳����___(�����)��
a.CO32- b.ClO- c.CH3COO- d.HCO3-
��3��������ˮϡ��0.1mol/L�Ĵ��ᣬ���и�ʽ��ʾ����ֵ��ˮ�������Ӷ��������___(�����)��
a.![]() b.
b.![]()
c. ![]() d.
d.![]()
��4�������£�0.1mol/LNaHCO3��Һ��pH����8������Һc(H2CO3)___c(CO32-)(���������=��)��ԭ����___��
��5��25��ʱ�������CH3COOH��CH3COONa�Ļ����Һ��pH=6������Һ�У�
��![]() =___(������ȷ��ֵ������ͬ)��
=___(������ȷ��ֵ������ͬ)��
��c(CH3COO-)-c(Na+)=___mol/L��