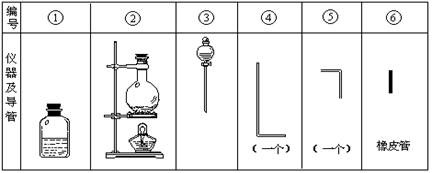
��Ŀ����
KMnO4��Һ������������ԭ��Ӧ�ζ��ı�Һ�仹ԭ����ΪMn2+������KMnO4��ǿ�����ԣ�������Һ�����ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����������������MnO(OH)2���������KMnO4����Һ�IJ���������ʾ��
�ٳ�ȡ�Զ�����������KMnO4��������ˮ�У�����Һ���Ȳ�������1 h�������ײ���©�����˳�ȥ���ܵ�MnO(OH)2���۹��˵õ�KMnO4��Һ�����棻������������ԭ�ζ���������70��80 ���������û��Լ�(���ȸߡ���Է��������ϴ��ȶ��ԽϺõ�����)��Һ�궨��Ũ�ȡ�
��ش��������⣺
(1)���ƺõ�KMnO4��Һ���淽���� ��ȷ��ȡһ�������KMnO4��Һ��Ҫʹ�õ�������____________��
(2)�����������У����ڱ궨KMnO4��Һ�Ļ��Լ����ѡ��________(�����)��
| A��H2C2O4��2H2O | B��FeSO4 | C��Ũ���� | D��Na2SO3 |
(4)���÷������ܵ�KMnO4����Һȥ�ⶨˮ����Fe2���ĺ�������õ�Ũ��ֵ��________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��ԭ���� ��KMnO4��Fe2����Ӧ�����ӷ���ʽΪ ��
(1) ��������ɫ�Լ�ƿ�����ڰ��� ��ʽ�ζ��� (2)A (3)  ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��(4)ƫ�� �����л�ԭ�����ʵ����ã�ʹKMnO4��Һ��Ũ�ȱ�С�����¼��������c(Fe2��)������ 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O
��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ��(4)ƫ�� �����л�ԭ�����ʵ����ã�ʹKMnO4��Һ��Ũ�ȱ�С�����¼��������c(Fe2��)������ 5Fe2����MnO4-��8H��=5Fe3����Mn2����4H2O
�������������(1) ����KMnO4�����ֽ⣬����KMnO4��Һ���淽������������ɫ�Լ�ƿ�����ڰ���������KMnO4��Һ����ǿ�����ԣ��ܸ�ʴ��ʽ�ζ����¶˵���Ƥ�ܲ����ü�ʽ�ζ�����ȡ��Ӧ����ʽ�ζ�����ȡ��(2) ����FeSO4��Na2SO3�ڿ��������������ʣ�Ũ�����ӷ����궨�궨KMnO4��ҺŨ��ʱ��������H2C2O4��2H2O �����ȶ�����ѡA����3��H2C2O4��KMnO4��Һ��Ӧ��H2C2O4������ΪCO2��KMnO4����ԭΪMn2+�����ݵ����غ�Ĺ�ϵʽ��5H2C2O4����2KMnO4����������ݴ������ɵ�KMnO4��Һ�����ʵ���Ũ��Ϊ mol��L��1���ζ��յ���ɫ�仯Ϊ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ����4��KMnO4����Һ���ã������ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����ʹŨ�Ƚ��ͣ��ⶨˮ����Fe2���ĺ�������õ�Ũ��ֵ��ƫ�ߣ����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
mol��L��1���ζ��յ���ɫ�仯Ϊ��Һ����ɫ��dz��ɫ���Ұ�����ڲ���ɫ����4��KMnO4����Һ���ã������ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����ʹŨ�Ƚ��ͣ��ⶨˮ����Fe2���ĺ�������õ�Ũ��ֵ��ƫ�ߣ����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
���㣺����ҩƷ�ı��桢�ζ�ʵ�����ӷ���ʽ��д��ϵʽ�����㡣

������һ����Ҫ�Ļ���ԭ�ϣ��㷺����ҩ���������߷��Ӻϳɵȹ�ҵ�����ᾧ�����ȵ�100��ʱʧȥ�ᾧˮ����Ϊ��ˮ���ᡣijѧϰС���ͬѧ���Ը�����Ϊԭ����ˮ�⡪������ˮ��ѭ��������ȡ���ᡣ
|
|
 �����������Ϣ�ش��������⣺
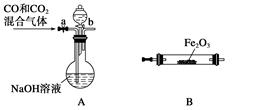
�����������Ϣ�ش��������⣺��ͼʾ�٢ڵ�������ˮ�����������ͼ1��װ���н��еģ�ָ��װ��A������ ��
��ͼʾ�٢ڵ�������ˮ������У���������������Ӧ��ʱ�����������ͬ������£��ı䷴Ӧ�¶��Կ��췴Ӧ�¶ȶԲ������ʵ�Ӱ�죬�������ͼ2��ʾ����ѡ����ѵķ�Ӧ�¶�Ϊ ��Ϊ�˴ﵽͼ2��ʾ���¶ȣ�ѡ��ͼ1��ˮԡ���ȣ����ŵ��� ��
����ͼʾ�ۢ��еIJ����漰�����ˣ�ϴ�ӡ���������й�˵����ȷ���� ��
A.��ʵ������У�ͨ��������ȴ������Һ�����Եõ��ϴ�ľ�����������ڳ���
B.��ϴ�ӳ���ʱ��Ӧ��Сˮ��ͷ��ʹϴ�Ӽ�����ͨ��������
C.Ϊ�˼���ϴ���Ƿ���ȫ��Ӧ��������ƿ�밲ȫƿ֮����Ƥ�ܣ�������ƿ�Ͽڵ���������Һ���Թ��н������ʵ�顣
D.Ϊ�˵õ�����ľ��壬����ѡ����������ֱ�Ӽ��ȣ����ڸ���������ȴ��
��Ҫ�ⶨ���ᾧ�壨H2C2O4��2H2O���Ĵ��ȣ���ȡ7.200g�Ʊ��IJ��ᾧ����������ˮ���250mL��Һ��ȡ25.00mL������Һ����ƿ�У���0.1000mol/L���Ը��������Һ�ζ�
��5H2C2O4+2MnO4��+6H+��2Mn2++10CO2��+8H2O����
��ȡ25.00mL������Һ�������� ��
���ڲ��ᴿ�Ȳⶨ��ʵ������У�����˵����ȷ���ǣ� ��
A.��ϴ�ζ���ʱ��Ӧ�ӵζ����Ͽڼ������������Һ��ʹ�ζ����ڱڳ����ϴ
B.��Һ��ȡ������Һʱ���轫���촦��Һ�崵����ƿ����ʹʵ�����ƫ��
C.�ζ�ʱ�������������ڿ�ס���������Ŀ���������������ʹ���ɶ�©����Һ
D.�ζ��յ��ȡ�ζ��̶ܿ�ʱ�����ӱ�ҺҺ�棬��ʹʵ�����ƫ��
���жϵζ��Ѿ��ﵽ�յ�ķ����ǣ� ��
�ܴﵽ�ζ��յ�ʱ�����ĸ��������Һ��20.00mL������ᾧ��Ĵ���Ϊ ��
�к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
(1)����ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��_________ _������֮�⣬װ���е�һ�����Դ����� ��
(2)Ϊ��֤��ʵ��ɹ���ͬѧ��ȡ�������ʩ����ͼ����ֽ������������________ ___��
(3)������60mL 0.25mol��L-1 H2SO4��50mL 0.55mol��L-1 NaOH��Һ���з�Ӧ������ʵ����ȣ����ų������� �����ȡ���������ȡ�������ʵ���������ȷ���������к��� ���ȡ�������ȡ���
(4)����NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز�������������
B����������������
C��һ��Ѹ�ٵ���
(5)ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ���
B���ҿ�ӲֽƬ�ò���������
C����������ձ�
D���������¶ȼ��ϵĻ��β���������ؽ���
(6)ʵ���������±���
������д�±��еĿհף�
| �¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к��Ȧ�H��______ ____ ( ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)____ ____��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
ijNa2CO3��Ʒ�л���һ������Na2SO4 (��������ᾧˮ����ij��ѧ��ȤС��������ַ����ⶨ����Ʒ��Na2CO3�������������Իش��������⡣
����һ���������з������Na2CO3���������Ĝy��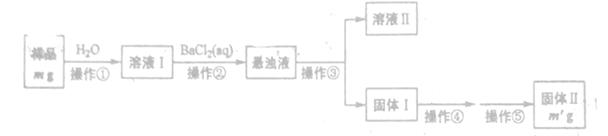
(1)�����ۺܵ͢����Ʒֱ�Ϊ_______��
(2)���������١����У�ʹ�õ�����������______(��������)��
(3)�жϲ����ڷ���ɵķ�����______
��������������ͼʵ��װ�ã��г�������ʡ�ԣ�.ѡ�������Լ�: a.Ũ����b.����NaHCO3��ҺC.6mol/L����D.2mol/L����, e.��ʯ��f. ��ˮCaCl2,�y����Ʒ��Na2CO3,������������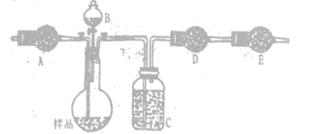
(4)��д���пո�
| ���� | �Լ� | ������Լ���Ŀ�� |
| A | | �������ʱϴȥCO2 |
| B | | ʹ��Ʒ��ַ�Ӧ�ų����� |
| C | a | |
| D | e | �������CO2 |
| E | e | |
�����й�ʵ��װ�ý��е���Ӧʵ�飬�ܴﵽʵ��Ŀ�ĵ���
ͼ1 ͼ2 ͼ3 ͼ4
| A����ͼ1��ʾװ�ó�ȥCl2�к��е�����HCl |
| B����ͼ2��ʾװ������NH4Cl������Һ�Ʊ�NH4Cl���� |
| C����ͼ3��ʾװ����ȡ����������CO2���� |
| D����ͼ4��ʾװ�÷���CCl4��ȡ��ˮ���ѷֲ���л����ˮ�� |

 ��
��