��Ŀ����
�ϳɰ���ҵ�ķ�ӦΪ��N2��g��+3H2��g��?2NH3��g������H��0��ҵ�ϳɰ�����ʾ��ͼ��ͼ1��ʾ��
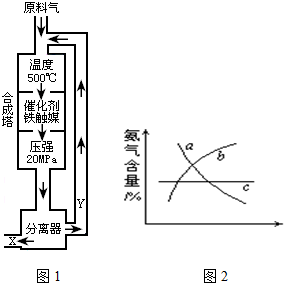
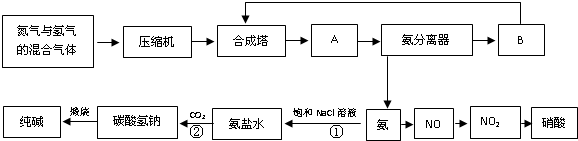
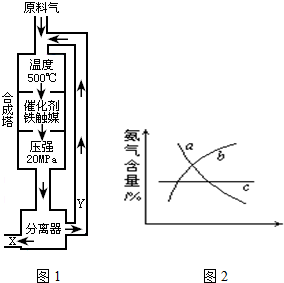
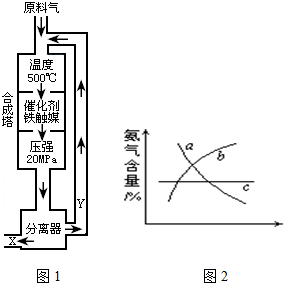
��1��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=______�������¶����ߣ�Kֵ______��������С�����䣩
��2��Y����Ҫ�ɷ�Ϊ______��
��3��������������ѡ������Ҫԭ���ǣ�ѡ����ĸ��ţ�______��
A���¶ȡ�ѹǿ�Ի�ѧƽ���Ӱ�죻
B������ý�ڸ��¶�ʱ���Դ�
C����ҵ�����ܶ��������ϡ��豸�����������ƣ�
��4���ı䷴Ӧ��������ʹƽ�ⷢ���ƶ�����ͼ2���ʾ�������ı䣬�����İٷֺ����ı仯���ƣ�
��������Ϊѹǿʱ���仯������ȷ���ǣ�ѡ����ĸ��ţ�______��
��������Ϊ�¶�ʱ���仯������ȷ���ǣ�ѡ����ĸ��ţ�______��
��5���ϳɰ�������ͨ���ⶨ��Ӧǰ��Ļ�������ܶ���ȷ������ת���ʣ�ij������úϳ�����N2��H2���ܶ�Ϊ0.5536g/L����״�������Ӻϳ����г����Ļ�������ܶ�Ϊ0.693g/L����úϳɰ���N2��ת����Ϊ���٣���
�⣺��1������ƽ�ⳣ������дҪ�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=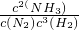 ���¶����ߣ�ƽ�������ƶ���c��NH3����С��c��N2����c��H2����������ƽ�ⳣ����С���ʴ�Ϊ��
���¶����ߣ�ƽ�������ƶ���c��NH3����С��c��N2����c��H2����������ƽ�ⳣ����С���ʴ�Ϊ��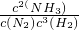 ����С��
������
��2��Y����Ҫ�ɷ�ΪNH3������N2��H2���ʴ�Ϊ��N2��H2��
��3����Ҫ�ӿ컯ѧ��Ӧ���ʼ�ƽ�������ƶ���Ӧѡ����¡���ѹ������������������ý��500��ʱ�������ѹ������ƽ�������ƶ������Զ��������ϡ��豸������Ҫ��ߣ�����ѹǿ����̫�ʴ�Ϊ��BC��
��4����������Ϊѹǿʱ����Ϊ����ѹǿ��ƽ�������ƶ���NH3�������仯������ȷ����b����������Ϊ�¶�ʱ����Ϊ��Ӧ������ȣ����£�ƽ�������ƶ���NH3�������ͣ��仯������ȷ����a���ʴ�Ϊ��b��a
��5���跴Ӧǰ��������е��������ʵ�������Ϊx���������ʵ�������Ϊ��1-x����������ã�
28x+2��1-x��=0.5536��22.4 ���x=0.4
����N2��H2�����ʵ���֮��Ϊ2��3
����ʼN2�����ʵ���Ϊ2mol��H2Ϊ3 mol��N2��ת����Ϊy
N2 +3H2 ?2NH3
2 3 0
y 3y 2y
2-y 3-3y 2y
�����ʵ���=2-y+3-3y+2y=��5-2y��mol
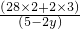 =0.693��22.4�����Ҷ��Ƿ�Ӧ�������Ħ��������
=0.693��22.4�����Ҷ��Ƿ�Ӧ�������Ħ��������
y=0.5 mol
����N2��ת����Ϊ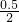 ��100%=25%���ʴ�Ϊ��25%��
��100%=25%���ʴ�Ϊ��25%��
��������1��ƽ�ⳣ��ָ������Ũ�ȵ�ϵ������֮���뷴Ӧ��Ũ��ϵ������֮���ı�ֵ�������¶����ߣ�ƽ�������ƶ����жϣ�
��2�����ݸ÷�ӦΪ���淴Ӧ�Լ�Y��ѭ��ʹ�ã�
��3��������������Ի�ѧ��Ӧ���ʼ�ƽ���Ӱ�죬ͬʱ��Ҫ����ʵ�������
��4����������ѹǿ��ƽ�������ƶ���NH3���������������¶ȣ�ƽ�������ƶ���NH3�������ͣ�
��5���ȸ���M=��Vm�����������ƽ��Ħ������������ʮ�ֽ��淨���㷴Ӧǰ���������N2��H2������ȣ�Ȼ�����M=��Vm=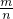 ���ת���ʣ�
���ת���ʣ�
������������Ҫ����ƽ�ⳣ���ĸ��ƽ���ƶ����ж�����㣬�ѶȲ���ע������ʽ���ⷨ�����ã�
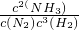 ���¶����ߣ�ƽ�������ƶ���c��NH3����С��c��N2����c��H2����������ƽ�ⳣ����С���ʴ�Ϊ��
���¶����ߣ�ƽ�������ƶ���c��NH3����С��c��N2����c��H2����������ƽ�ⳣ����С���ʴ�Ϊ��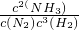 ����С��
����С����2��Y����Ҫ�ɷ�ΪNH3������N2��H2���ʴ�Ϊ��N2��H2��
��3����Ҫ�ӿ컯ѧ��Ӧ���ʼ�ƽ�������ƶ���Ӧѡ����¡���ѹ������������������ý��500��ʱ�������ѹ������ƽ�������ƶ������Զ��������ϡ��豸������Ҫ��ߣ�����ѹǿ����̫�ʴ�Ϊ��BC��
��4����������Ϊѹǿʱ����Ϊ����ѹǿ��ƽ�������ƶ���NH3�������仯������ȷ����b����������Ϊ�¶�ʱ����Ϊ��Ӧ������ȣ����£�ƽ�������ƶ���NH3�������ͣ��仯������ȷ����a���ʴ�Ϊ��b��a
��5���跴Ӧǰ��������е��������ʵ�������Ϊx���������ʵ�������Ϊ��1-x����������ã�
28x+2��1-x��=0.5536��22.4 ���x=0.4
����N2��H2�����ʵ���֮��Ϊ2��3
����ʼN2�����ʵ���Ϊ2mol��H2Ϊ3 mol��N2��ת����Ϊy
N2 +3H2 ?2NH3
2 3 0
y 3y 2y
2-y 3-3y 2y
�����ʵ���=2-y+3-3y+2y=��5-2y��mol
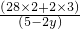 =0.693��22.4�����Ҷ��Ƿ�Ӧ�������Ħ��������
=0.693��22.4�����Ҷ��Ƿ�Ӧ�������Ħ��������y=0.5 mol
����N2��ת����Ϊ
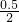 ��100%=25%���ʴ�Ϊ��25%��
��100%=25%���ʴ�Ϊ��25%����������1��ƽ�ⳣ��ָ������Ũ�ȵ�ϵ������֮���뷴Ӧ��Ũ��ϵ������֮���ı�ֵ�������¶����ߣ�ƽ�������ƶ����жϣ�
��2�����ݸ÷�ӦΪ���淴Ӧ�Լ�Y��ѭ��ʹ�ã�
��3��������������Ի�ѧ��Ӧ���ʼ�ƽ���Ӱ�죬ͬʱ��Ҫ����ʵ�������
��4����������ѹǿ��ƽ�������ƶ���NH3���������������¶ȣ�ƽ�������ƶ���NH3�������ͣ�
��5���ȸ���M=��Vm�����������ƽ��Ħ������������ʮ�ֽ��淨���㷴Ӧǰ���������N2��H2������ȣ�Ȼ�����M=��Vm=
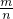 ���ת���ʣ�
���ת���ʣ�������������Ҫ����ƽ�ⳣ���ĸ��ƽ���ƶ����ж�����㣬�ѶȲ���ע������ʽ���ⷨ�����ã�

��ϰ��ϵ�д�
�����Ŀ