��Ŀ����
MMA����һ�ָ߷��ӻ�����л��������ĵ��壬������ȡ����ϩ������ȵ���Ҫԭ�ϡ����������Ʊ��߷��ӣ�MMA����;�������������磺

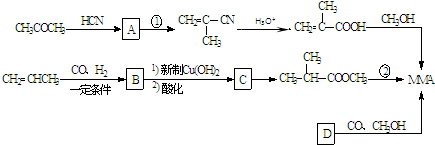
��1����ҵ�Ͻ�A�ͼ״�����������һ��һ����Ӧ����MMA���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
��2����Ӧ������500�沢�д������ڵ������·����ģ����䷴Ӧ����Ϊ��____________��
��3��ij���ʼ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��������л�������̼ԭ�ӣ�����
 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��_____________��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��_____________��
��4������D�ĺ˴Ź��������������壬����CO��CH3OH�����ʵ���֮��1�U1�U1��Ӧǡ������MMA����D�Ľṹ��ʽΪ_______________���÷������ŵ���_______________��
��5��MMA��1-������Ӧ��ȡ����ϩ������Ļ�ѧ����ʽΪ��____________________��
��6�����������������ṩ������Ϣ��д����(CH3)2C=CH2�Ʊ�������
��2����Ӧ������500�沢�д������ڵ������·����ģ����䷴Ӧ����Ϊ��____________��
��3��ij���ʼ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��������л�������̼ԭ�ӣ�����
 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��_____________��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��_____________����4������D�ĺ˴Ź��������������壬����CO��CH3OH�����ʵ���֮��1�U1�U1��Ӧǡ������MMA����D�Ľṹ��ʽΪ_______________���÷������ŵ���_______________��
��5��MMA��1-������Ӧ��ȡ����ϩ������Ļ�ѧ����ʽΪ��____________________��
��6�����������������ṩ������Ϣ��д����(CH3)2C=CH2�Ʊ�������

�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ������

____________________________
��1��(CH3)2C(OH)CN+CH3OH+H2SO4��CH2��C(CH3)COOCH3+NH4HSO4
��2����ȥ���������ѽ�
��3��HCOOCH(CH3)CH2CH3
��4��CH��CCH3��ԭ��������Ϊ100%
��2����ȥ���������ѽ�
��3��HCOOCH(CH3)CH2CH3
��4��CH��CCH3��ԭ��������Ϊ100%
��5��
��6��


��6��


��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ
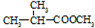 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��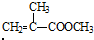 +CH3CH2CH2OH��
+CH3CH2CH2OH��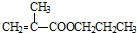 +CH3OH
+CH3OH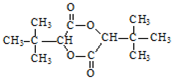 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��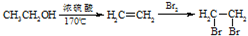

 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��__ __
��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��__ __
��


 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��__ __
��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��__ __
��
