��Ŀ����
ijѧ����һ����Է�������Ϊ204.0�Ķ�Ԫ�����ʽ�����ⶨNaOH��Һ��Ũ�ȣ���NaOH��Һ��Ũ����0.1 mol��L-1���ң��ζ��յ�ʱ��Һ��pHԼΪ9.1��(1)������������ƽ��������ʱ�������̷���һ��1 g �����룬����λ������ͼ��ʾ��

(2)���ƺõ��η�����ƿ�У�������ˮ�ܽ⣬��Һ��ɫ���ټ���ָʾ��_________________(�ӷ�̪��ʯ����ѡ��)1��2�Σ���NaOH��Һ�ζ����յ㣬������________________________��
(3)������������ʵ�飬��ȡ������������ͬ����д�±���
ʵ���� | ��ʽ�ε�����/g | ����NaOH��Һ���/mL |
1 |
| 18.2 |
2 |
| 17.1 |
3 |
| 16.9 |
(4)�ζ������ϴ���ǵ�________��ʵ�飬����������Ŀ���ԭ����(ֻ��д���ּ���)��
��_____________________________________________________________��
��_____________________________________________________________��
��_____________________________________________________________��
(5)NaOH��Һ�����ʵ���Ũ��Ϊ___________________(ֻ�г�����ʽ������������)��
(2)��̪ ��ɫ���dz��ɫ����0.5 min�ڲ���ɫ
(3)0.4 0.4 0.4
(4)1 ��ʢװNaOH��Һ�ĵζ���δ��NaOH��Һ��ϴ��ֻ��ˮϴ����������NaOH��Һ���ƫ�ڵζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ���۵ζ��յ��жϲ���ʹ��Һ�ʺ�ɫ���ܵζ�ʱ�еζ�Һ������ƿ�⣻�ݶ��յ����ʱ���������ӵȵȡ�(��д��������)
(5)0.12 mol��L-1
������(1)������ƽ�ij���ԭ���Ǹܸ�ƽ��ԭ������������ƽ����ʱ�������ڱ�����������������Ǽӵ������ϵģ��������ԭ��Ϊ�������롣�ֽ�����������������ϣ���������������ϣ������ڱ����ָʾ������Ϊ0.6 g����m(������)+m(����)=m(����)����m(������)+0.6 g=1 g��m(������)=0.4 g��
(2)��ζ��յ�ʱ��Һ��pHԼΪ9.1����̪�ı�ɫ��Χ��pH=8��10����ʯ��ı�ɫ��Χ��pH=5��8����Ӧѡ�÷�̪��ָʾ���������Ǽ�ζ��������ʣ��ʵζ��յ������Ӧ����ɫ���dz��ɫ����0.5 min�ڲ���ɫ��
(3)�������ӦΪ(1)��m(������)=0.4 g��
(4)�ӱȽϱ�����������NaOH��Һ��������Կ������������������������С������һ������ֵ�������������ֵ�нϴ��ƫ��ʵζ������ϴ��Ӧ�ǵ�һ�Ρ���һ��������ֵ���Դ��ڶ������Σ�����������Ŀ���ԭ���У���ʢװNaOH��Һ�ĵζ���δ��NaOH��Һ��ϴ��ֻ��ˮϴ����������NaOH��Һ���ƫ�ڵζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ���۵ζ��յ��жϲ���ʹ��Һ�ʺ�ɫ���ܵζ�ʱ�еζ�Һ������ƿ�⣻�ݶ��յ����ʱ���������ӵȵȡ�
(5)������NaOH��Ӧʱ�����ʵ�����Ϊ1��1������n���=n���Σ�����c(NaOH)��
V(NaOH)=![]()
V(NaOH)=![]() ��10-3 L(���һ������NaOH��Һ��������ϴʲ���)
��10-3 L(���һ������NaOH��Һ��������ϴʲ���)
���ǣ�c(NaOH)=![]() mol��L-1=0.12 mol��L-1
mol��L-1=0.12 mol��L-1

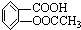 ����Է�������Ϊ180����
����Է�������Ϊ180����
