��Ŀ����
�����ŷ�CO2����ɡ�����ЧӦ����Ϊ�˼���úȼ�նԻ�����ɵ���Ⱦ��ú�������Ǹ�Ч���������ú̿����Ҫ;����ú�ۺ����õ�һ��;����ͼ��ʾ��

��1����֪��C(s) �� H2O(g) = CO(g)��H2(g) ��H1����131.3 kJ��mol��1
��C(s) �� 2H2O(g) = CO2(g) �� 2H2(g) ��H2����90 kJ��mol��1
��һ����̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ�� ________________________��
��2������ͼԭ���װ�ÿ�����ɹ��̢ݵ�ת������װ��b�缫�ĵ缫��Ӧʽ��_______________________��

��3����ѹǿΪ0.1 MPa�����£��ݻ�ΪV L���ܱ�������a mol CO��2a mol H2�ڴ��������·�Ӧ���ɼ״���
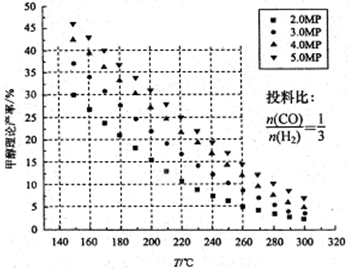
CO(g)��2H2(g) CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����

��p1________p2(�����������������)��
���������������������£���������������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ƽ��ת����________(���������С�����䡱)��
����p1�£�100 ��ʱ��CO(g)��2H2(g) CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
��4����ͼ��ʾCO2��H2��Ӧ����CH3OH��H2O�Ĺ���������(��λΪkJ��mol��1)�ı仯��

���ڸ÷�Ӧ������˵���У���ȷ����________(����)��
A����H��0����S��0 B����H��0����S��0
C����H��0����S��0 D����H��0����S��0
��5��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��

�ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)��________��
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����________(����)��
A�������¶� B����CH3OH(g)��ʱҺ���Ƴ�
C��ѡ���Ч���� D���ٳ���1 mol CO2��3 mol H2

��1����֪��C(s) �� H2O(g) = CO(g)��H2(g) ��H1����131.3 kJ��mol��1
��C(s) �� 2H2O(g) = CO2(g) �� 2H2(g) ��H2����90 kJ��mol��1
��һ����̼��ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ�� ________________________��
��2������ͼԭ���װ�ÿ�����ɹ��̢ݵ�ת������װ��b�缫�ĵ缫��Ӧʽ��_______________________��

��3����ѹǿΪ0.1 MPa�����£��ݻ�ΪV L���ܱ�������a mol CO��2a mol H2�ڴ��������·�Ӧ���ɼ״���
CO(g)��2H2(g)
 CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
��p1________p2(�����������������)��
���������������������£���������������a mol CO��2a mol H2���ﵽ��ƽ��ʱ��CO��ƽ��ת����________(���������С�����䡱)��
����p1�£�100 ��ʱ��CO(g)��2H2(g)
 CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)��
CH3OH(g)��Ӧ��ƽ�ⳣ��Ϊ________(�ú�a��V�Ĵ���ʽ��ʾ)����4����ͼ��ʾCO2��H2��Ӧ����CH3OH��H2O�Ĺ���������(��λΪkJ��mol��1)�ı仯��

���ڸ÷�Ӧ������˵���У���ȷ����________(����)��
A����H��0����S��0 B����H��0����S��0
C����H��0����S��0 D����H��0����S��0
��5��Ϊ̽����Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1 L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)
 CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
CH3OH(g)��H2O(g)�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��������ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)��________��
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����________(����)��
A�������¶� B����CH3OH(g)��ʱҺ���Ƴ�
C��ѡ���Ч���� D���ٳ���1 mol CO2��3 mol H2
��1�� CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.3 kJ��mol��1����2�� O2 +4e�� +2H2O = 4OH���� ��3���٣��� V 2 / a2���������� ��4��C�� ��5����0.075 mol/( L��min). ��BD
�����������1����-�ٿɵã�CO(g)��H2O(g)=CO2(g)��H2(g)����H����41.3 kJ/mol.. ��2����ȼ�ϵ���У�ͨ��ȼ�ϵĵ缫��������ͨ�������ĵ缫��������a�缫�Ǹ�����b�缫��������b�缫�ĵ缫��Ӧʽ��O2 +4e�� +2H2O = 4OH������3�� ����ͼ���Կ��������¶���ͬʱ��ת����P2>P1������ƽ���ƶ�ԭ�����������������������¡�����ѹǿ����ѧƽ�������������С�ķ����ƶ�����������Ӧ�����ƶ�����ʱ��Ӧ���ת������ߡ�����P1<P2. ���������������������£���������������a mol CO��2a mol H2������������ϵ��ѹǿ����ʱ��ѧƽ��������Ӧ�����ƶ����ʴﵽ��ƽ��ʱ��CO��ƽ��ת����������p1�£�100 ��ʱ��CO(g)��2H2(g)
 CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g��
CH3OH(g)��Ӧ��ƽ�ⳣ��ΪK="C" (CH3OH)/ { C(CO)��C2(H2)} ="(" a/2V)��{(a/2V) ��(a/V)}2=" V" 2 / a2.��4��CO2(g)+3H2��g�� CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��
CH3OH(g)+CO(g).��ͼ�ɿ����÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���÷�Ӧ������Ӧ�Ǹ����������С�ķ��ȷ�Ӧ�����ԡ�H<0����S<0��ѡ��Ϊ��C����5���ٴӷ�Ӧ��ʼ��ƽ�⣬CO2��ƽ����Ӧ����v(CO2)=��1-0.25��mol/L��10min="0.075" mol/( L��min). ��A�����¶Ȼ�ѧƽ�������ȷ����ƶ������ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ���������¶Ȼ�ѧƽ�����淴Ӧ�����ƶ���B����С�������Ũ�ȣ���ѧƽ��������Ӧ�����ƶ����ʽ�CH3OH(g)��ʱҺ���Ƴ���ʹƽ��������Ӧ�����ƶ���C������ �Ի�ѧƽ����Ӱ�졣D���ﵽƽ��ʱ���ٳ���1 mol CO2��3 mol H2����������ѹǿ����ѧƽ�������������С�ķ�������Ӧ�����ƶ���������ʹ��ѧƽ��������Ӧ�����ƶ��Ĵ�ʩ��BD��
��ϰ��ϵ�д�
 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
�����Ŀ
 =" +28.7" kJ��mol-1
=" +28.7" kJ��mol-1 2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��
2NO(g) ��H����֪�÷�Ӧ�� T ��ʱ��ƽ�ⳣ��K��9.0��


 mol;
mol;

 ���>������<����=����
���>������<����=���� 2SO3(g) ��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
2SO3(g) ��H��0��Ӧ��ij�¶��£�SO2��ƽ��ת���ʣ���������ϵ��ѹǿ��p���Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺


 CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺