��Ŀ����
����1mol/L�İ�ˮ������������ȷ���ǣ�������Һ���ʱ������仯��
- A.����״����22.4L��������1Lˮ�������Һ�����ɵõ�1mol/L�İ�ˮ
- B.1mol/L�İ�ˮ����������С��1.7%
- C.��1mol/L�İ�ˮ��ˮ��������Ϻ����ð�ˮ�����ʵ���Ũ�ȴ���0.5mol/L
- D.��������Ϊ10%�İ�ˮ����������Ϊ20%�İ�ˮ�������ϣ����ð�ˮ��������������15%
C
������A������״����22.4L���������ʵ���Ϊ1mol������ˮ���1L��Һ��Ũ��Ϊ1mol/L��
B����ˮ���ܶ�С��1g/ml������c=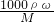 �����жϣ�
�����жϣ�
C����ˮ��Ũ��Խ���ܶ�ԽС����Ϻ���Һ������Ϊԭ��2������Ϻ���Һ���ܶȴ���1mol/L��ˮ��Һ���ܶȣ����Ի�Ϻ���Һ�����С��1mol/L��ˮ��Һ�������2����
D������Һ���Ϊ1ml���ٶ���������Ϊ10%�İ�ˮ�ܶ�Ϊx����������Ϊ20%�İ�ˮ���ܶ�Ϊy����ʾ����Ϻ�ˮ��������������ϰ�ˮŨ��Խ���ܶ�ԽС�����жϣ�
���A������״����22.4L���������ʵ���Ϊ1mol������ˮ���1L��Һ��Ũ��Ϊ1mol/L�����1L��ָ��Һ������������ܼ����������A����
B���ˮ�ܶ�Ϊ��g/ml������c=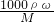 ��֪����=
��֪����=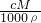 =
=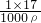 =1.7%
=1.7% �����ڰ�ˮ�ܶȦѣ�1�����Ԧ�=1.7%
�����ڰ�ˮ�ܶȦѣ�1�����Ԧ�=1.7% ��1.7%����B����
��1.7%����B����
C����ˮ��Ũ��Խ���ܶ�ԽС����Ϻ���Һ������Ϊԭ��2������Ϻ���Һ���ܶȴ���1mol/L��ˮ��Һ���ܶȣ����Ի�Ϻ���Һ�����С��1mol/L��ˮ��Һ�����2�������ʰ������ʵ������䣬�ʻ�Ϻ����ʵ���Ũ�ȴ���0.5mol/L����C��ȷ��
D������Һ���Ϊ1ml���ٶ���������Ϊ10%�İ�ˮ�ܶ�Ϊx����������Ϊ20%�İ�ˮ���ܶ�Ϊy�����Ϻ�ˮ������������=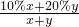 =10%+10%
=10%+10%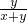 =10%+10%
=10%+10%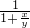 ����ˮŨ��Խ���ܶ�ԽС������x��y����
����ˮŨ��Խ���ܶ�ԽС������x��y����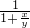 ��
�� ������10%+10%
������10%+10%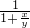 ��15%����D����
��15%����D����
��ѡC��
���������⿼�����ʵ���Ũ�ȼ��㣬�Ѷ��еȣ�ע�ʽ��������ã��ؼ��������ʵ���Ũ�ȶ��壬ע������ܽ���ܶȱ�ˮ������ֲ�ͬŨ����Һ��ϣ��������Ϻ�������Һ�����ʵ������������ڵ�������Ϻ�������Һ�����ʵ�������������������Һ�����ʵ���������֮�͵�һ�룩�����������ơ��Ȼ�����Һ�ȣ�ͬ���У����ܶȱ�ˮС�����ֲ�ͬŨ����Һ��ϣ��������Ϻ�������Һ�����ʵ���������С�ڵ�������Ϻ�������Һ�����ʵ�������������������Һ�����ʵ���������֮�͵�һ�룩���簱ˮ���ƾ���Һ�ȣ�
������A������״����22.4L���������ʵ���Ϊ1mol������ˮ���1L��Һ��Ũ��Ϊ1mol/L��
B����ˮ���ܶ�С��1g/ml������c=
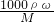 �����жϣ�
�����жϣ�C����ˮ��Ũ��Խ���ܶ�ԽС����Ϻ���Һ������Ϊԭ��2������Ϻ���Һ���ܶȴ���1mol/L��ˮ��Һ���ܶȣ����Ի�Ϻ���Һ�����С��1mol/L��ˮ��Һ�������2����
D������Һ���Ϊ1ml���ٶ���������Ϊ10%�İ�ˮ�ܶ�Ϊx����������Ϊ20%�İ�ˮ���ܶ�Ϊy����ʾ����Ϻ�ˮ��������������ϰ�ˮŨ��Խ���ܶ�ԽС�����жϣ�
���A������״����22.4L���������ʵ���Ϊ1mol������ˮ���1L��Һ��Ũ��Ϊ1mol/L�����1L��ָ��Һ������������ܼ����������A����
B���ˮ�ܶ�Ϊ��g/ml������c=
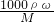 ��֪����=
��֪����=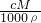 =
=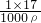 =1.7%
=1.7% �����ڰ�ˮ�ܶȦѣ�1�����Ԧ�=1.7%
�����ڰ�ˮ�ܶȦѣ�1�����Ԧ�=1.7% ��1.7%����B����
��1.7%����B����C����ˮ��Ũ��Խ���ܶ�ԽС����Ϻ���Һ������Ϊԭ��2������Ϻ���Һ���ܶȴ���1mol/L��ˮ��Һ���ܶȣ����Ի�Ϻ���Һ�����С��1mol/L��ˮ��Һ�����2�������ʰ������ʵ������䣬�ʻ�Ϻ����ʵ���Ũ�ȴ���0.5mol/L����C��ȷ��
D������Һ���Ϊ1ml���ٶ���������Ϊ10%�İ�ˮ�ܶ�Ϊx����������Ϊ20%�İ�ˮ���ܶ�Ϊy�����Ϻ�ˮ������������=
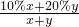 =10%+10%
=10%+10%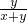 =10%+10%
=10%+10%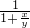 ����ˮŨ��Խ���ܶ�ԽС������x��y����
����ˮŨ��Խ���ܶ�ԽС������x��y����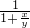 ��
�� ������10%+10%
������10%+10%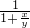 ��15%����D����
��15%����D������ѡC��
���������⿼�����ʵ���Ũ�ȼ��㣬�Ѷ��еȣ�ע�ʽ��������ã��ؼ��������ʵ���Ũ�ȶ��壬ע������ܽ���ܶȱ�ˮ������ֲ�ͬŨ����Һ��ϣ��������Ϻ�������Һ�����ʵ������������ڵ�������Ϻ�������Һ�����ʵ�������������������Һ�����ʵ���������֮�͵�һ�룩�����������ơ��Ȼ�����Һ�ȣ�ͬ���У����ܶȱ�ˮС�����ֲ�ͬŨ����Һ��ϣ��������Ϻ�������Һ�����ʵ���������С�ڵ�������Ϻ�������Һ�����ʵ�������������������Һ�����ʵ���������֮�͵�һ�룩���簱ˮ���ƾ���Һ�ȣ�

��ϰ��ϵ�д�
�����Ŀ
��1����25�桢101kPa�£�1g����ȼ������CO2��Һ̬ˮʱ����55.6kJ�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��2���±��е����ݱ�ʾ�ƻ�1mol��ѧ�������ĵ����������ݼ������ݼ������·�Ӧ�ķ�Ӧ�ȡ�H��
CH4��g��+4F2��g��=CF4��g��+4HF��g����H= ��
��3������������N2H4����Ϊȼ�ϣ�NO2����Ϊ�����������߷�Ӧ����N2��ˮ��������֪��
N2 ��g��+2O2 ��g��=2NO2 ��g����H1=+67.7kJ?mol-1��
2H2 ��g��+O2 ��g��=2H2O��g����H2=-484kJ?mol-1��
N2H4 ��g��+O2 ��g��=N2 ��g��+2H2O��g����H3=-534kJ?mol-1��
H2O��1��=H2O��g����H5=+44.0kJ?mol-1��
д��N2H4������NO2���巴Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽ��
��4������˵����ȷ���ǣ�����ţ� ��
A��ͬ�¶��£�0.1mol?L-1NH4Cl��Һ��NH4+��Ũ�ȱ�0.1mol?L-1��ˮ��NH4+��Ũ�ȴ�
B����ϡ����ϴ��AgCl��������ˮϴ�����AgClС��
C������Al��OH��3��s��?Al��OH��3��aq��?Al3+��aq��+3OH-��aq����ǰ��Ϊ�ܽ�ƽ�⣬����ǵ���ƽ�⣻
D����ȥ��Һ�е�Mg2+����OH-����Mg2+����CO32-Ч���ã�˵��Mg��OH��2���ܽ�ȱ�MgCO3�Ĵ�
��2���±��е����ݱ�ʾ�ƻ�1mol��ѧ�������ĵ����������ݼ������ݼ������·�Ӧ�ķ�Ӧ�ȡ�H��
CH4��g��+4F2��g��=CF4��g��+4HF��g����H= ��
| ��ѧ�� | C-H | C-F | H-F | F-F |
| ���� | 414 | 489 | 565 | 158 |
N2 ��g��+2O2 ��g��=2NO2 ��g����H1=+67.7kJ?mol-1��
2H2 ��g��+O2 ��g��=2H2O��g����H2=-484kJ?mol-1��
N2H4 ��g��+O2 ��g��=N2 ��g��+2H2O��g����H3=-534kJ?mol-1��
H2O��1��=H2O��g����H5=+44.0kJ?mol-1��
д��N2H4������NO2���巴Ӧ���ɵ�����Һ̬ˮ���Ȼ�ѧ����ʽ��
��4������˵����ȷ���ǣ�����ţ� ��
A��ͬ�¶��£�0.1mol?L-1NH4Cl��Һ��NH4+��Ũ�ȱ�0.1mol?L-1��ˮ��NH4+��Ũ�ȴ�
B����ϡ����ϴ��AgCl��������ˮϴ�����AgClС��
C������Al��OH��3��s��?Al��OH��3��aq��?Al3+��aq��+3OH-��aq����ǰ��Ϊ�ܽ�ƽ�⣬����ǵ���ƽ�⣻
D����ȥ��Һ�е�Mg2+����OH-����Mg2+����CO32-Ч���ã�˵��Mg��OH��2���ܽ�ȱ�MgCO3�Ĵ�
 ��ѧ��һֱ�����ڡ��˹��̵������·����о���
��ѧ��һֱ�����ڡ��˹��̵������·����о���