��Ŀ����
��ÿ��2�֣���14�֣�
��һ����̼���Ʒ�ĩ����0.2000 mol��L-1Na2CO3��Һ0.5L
��1����ѡ�õIJ�����������Ʒ ������ĸ��ţ�
B��0.5L ƽ����ƿ C��0.5L ����ƿ D��0.5LԲ����ƿ E���ձ� F���Թ� G����ͷ�ι� H����Ͳ I��������ƽ J��ҩ�� M����ֽ N��������
��2��Ӧ��ȡ g ̼���Ʒ�ĩ������ˮ
��������0.2000 mol��L-1Na2CO3��Һ�궨δ֪Ũ�ȵ�����
��1����0.2000 mol��L-1Na2CO3��Һװ�� (��ʽ����ʽ)�ζ����С��Ӵ˵ζ����зų�20. 00mL0.2000 mol��L-1Na2CO3��Һ����ƿ��,��2��3�μ�����ָʾ������δ֪Ũ�ȵ��������ζ�0.2000 mol��L-1Na2CO3��Һ������ �жϵζ��յ�ﵽ��
��2���յ�ﵽʱ����ȥ����16.00mL�����ε�ƽ��ֵ����������c(HCl)=
�������к��ȵIJⶨ
�ã�����ʵ���б궨��Ũ�ȵ�����50.0mL��0.55mol��L-1NaOH 50.0mL��Ӧ���к��ȵIJⶨʵ�顣ÿ��������һ��������ʵ�����¶����ߵ�ƽ��ֵΪ3.41�棬��ʵ�����к��ȡ�H= (��Ϻ���Һ�ı�����C = 4.18J����-1��g-1)��ʵ�����к��ȱ����� (ƫ�ߣ���ȣ�ƫ��)
(ÿ��2�� , ��14��)
��һ����1��CHENG ��2��10.6
��������1����ʽ ��ɫ��ɳ�ɫ30s����ȥ
��2��0.5mol/L
������- 57.0 KJ/mol ƫ��
��������

 �Ķ��쳵ϵ�д�
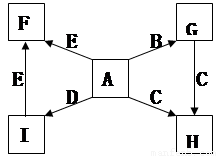
�Ķ��쳵ϵ�д�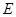 ���Է�����Ӧ��2E��I
���Է�����Ӧ��2E��I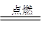 2F��D��F�е�EԪ�ص���������Ϊ60%.
2F��D��F�е�EԪ�ص���������Ϊ60%.