��Ŀ����
��ҵ����﮻�ʯ��Li2O?Al2O3?4SiO2��������Ca��MgԪ�أ�Ϊԭ������̼��ﮣ��䲿�ֹ����������£�

��֪����Li2O?Al2O3?4SiO2+H2SO4��Ũ��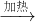 Li2SO4+Al2O3?4SiO2?H2O��
Li2SO4+Al2O3?4SiO2?H2O��
��ijЩ���ʵ��ܽ�ȣ�S�����±���ʾ��
| T/�� | 20 | 40 | 60 | 80 |
| S��Li2CO3��/g | 1.33 | 1.17 | 1.01 | 0.85 |
| S��Li2SO4��/g | 34.2 | 32.8 | 31.9 | 30.7 |

��1��������д���������ڼ����Լ��Ļ�ѧʽ______��______��
��2��������д�����������õ����ʵĻ�ѧʽ______��______��
��3��������з�Ӧ�����ӷ���ʽ��______��
��4����֪����2����Ҫ�ɷ���Mg��OH��2��CaCO3������Һ1�м���ʯ����������ǣ����û�ѧƽ��ԭ��������______��
��5������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ����______��
��6���������ڹ�ҵ�����������ͻ���ϣ����ͻ�ש�����������������챦ʯ�ȣ�ͬʱ������Ҳ��������ԭ�ϣ�д���������������Ļ�ѧ����ʽ��______��
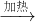 Li2SO4+Al2O3?4SiO2?H2O�����ƶ�������Al2O3?4SiO2?H2O������õ������������ȼ��������ܽ����������������費��Ӧ�����˵õ��Ȼ�����Һ���������һˮ�ϰ�����ȫ�������������������������������˵õ��������յõ����������ʴ�Ϊ��HCl��NH3?H2O��
Li2SO4+Al2O3?4SiO2?H2O�����ƶ�������Al2O3?4SiO2?H2O������õ������������ȼ��������ܽ����������������費��Ӧ�����˵õ��Ȼ�����Һ���������һˮ�ϰ�����ȫ�������������������������������˵õ��������յõ����������ʴ�Ϊ��HCl��NH3?H2O����2�����ݣ�1����ѡ�Լ��������ͼ�ж�Ϊ���������ܽ��õ��Ȼ�����Һ������һˮ�ϰ��õ���������������
�ʴ�Ϊ��AlCl3��Al��OH��3��
��3��������з�Ӧ�������ܽ������������Ȼ�����ˮ�ķ�Ӧ����Ӧ�����ӷ���ʽΪ��Al2O3+6H+=Al3++3H2O��
�ʴ�Ϊ��Al2O3+6H+=Al3++3H2O��
��4��ʯ�������������ƣ��ṩ���������Ӻ����ӣ�����ȫ�ij���þ���Ӻ�̼������ӣ�
�ʴ�Ϊ������Ca2+��OH-��Ũ�ȣ�������Mg��OH��2��CaCO3��������
��5������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ͼ����̼����ܽ�����¶�����С�����ٳ�������ʧ��
�ʴ�Ϊ��Li2CO3���ܽ�����¶����߶���С����ˮϴ�ӿɼ���Li2CO3����ʧ��
��6����ҵ�����ü������ʯ�����������ڵ��õ�������������Ӧ�Ļ�ѧ����ʽΪ��2Al2O3�����ڣ�
 4Al+3O2����
4Al+3O2�����ʴ�Ϊ��2Al2O3�����ڣ�
 4Al+3O2����
4Al+3O2������������1�����﮻�ʯ�ɷ֣���������ͼ��Ӧ��ϵ������֪������Al2O3?4SiO2?H2O���������ᣬ�������ܽ�Ϊ�Ȼ������������費��Ӧ�����˵õ���Һ�м���һˮ�ϰ����������������������յõ���������
��2����������ͼ��Ӧ��ϵ������֪������Al2O3?4SiO2?H2O���������ᣬ�������ܽ�Ϊ�Ȼ������������費��Ӧ�����˵õ���Һ�м���һˮ�ϰ����������������������յõ���������
��3��������з�Ӧ���������ܽ������������Ȼ�����ˮ��
��4��ʯ�������������ƣ��ṩ���������Ӻ����ӣ�����ȫ�ij���þ���Ӻ�̼������ӣ�
��5������ͼ��������֪̼����ܽ���� �¶�����С��
��6����ҵ�ϵ�����ڵ���������������������
���������⿼���������������̵ķ����жϣ����̷������������ʵ�Ӧ���ǽ���ؼ�����Ҫ�������仯�������ʵ�Ӧ�ã�����������ȡ��������Ŀ�Ѷ��еȣ�

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д���12�֣���ҵ����﮻�ʯ��Li2O��A12O3��4SiO2��������Ca��MgԪ�أ�Ϊԭ������̼��ﮡ��䲿�ֹ����������£�
��֪����Li2O��Al2O3��4SiO2 +H2SO4��Ũ�� Li2SO4+Al2O3��4SiO2��H2O��
Li2SO4+Al2O3��4SiO2��H2O��
��ijЩ���ʵ��ܽ�ȣ�S�����±���ʾ��
| T/�� | 20 | 40 | 60 | 80 |
| S(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
| S(Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |

��2����֪��Һ1�е���Ҫ����ΪLi+��Mg2����Ca2+��SO42��������2����Ҫ�ɷ���Mg(OH)2��CaCO3������Һ1�м���ʯ����������ǣ����û�ѧƽ��ԭ����������д���ӷ���ʽ��
__________________________________________________________________��
��3������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ���� ���� ��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������¡�
a����Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ����Ĥ�������ö��Ե缫��⡣
b��������LiOH��Һ�м������NH4HCO3��Һ�����ˡ���ɵøߴ�Li2CO3��
�� a�У������ĵ缫��Ӧʽ��______________________________________��
��b�У�����Li2CO3��Ӧ�Ļ�ѧ����ʽ��______________________________��
��12�֣���ҵ����﮻�ʯ��Li2O��A12O3��4SiO2��������Ca��MgԪ�أ�Ϊԭ������̼��ﮡ��䲿�ֹ����������£�

��֪���� Li2O��Al2O3��4SiO2
+H2SO4��Ũ�� Li2SO4+Al2O3��4SiO2��H2O��
Li2SO4+Al2O3��4SiO2��H2O��
�� ijЩ���ʵ��ܽ�ȣ�S�����±���ʾ��
|
T/�� |
20 |
40 |
60 |
80 |
|
S(Li2CO3)/g |
1.33 |
1.17 |
1.01 |
0.85 |
|
S(Li2SO4)/g |
34.2 |
32.8 |
31.9 |
30.7 |
��1��������1�з����Al2O3�IJ�����������ͼ��ʾ�����ű�ʾ������Լ��������ʾ���õ������ʡ����������ѷ�Ӧ�����ӷ���ʽ��____________________________��

��2����֪��Һ1�е���Ҫ����ΪLi+��Mg2����Ca2+��SO42��������2����Ҫ�ɷ���Mg(OH)2��CaCO3������Һ1�м���ʯ����������ǣ����û�ѧƽ��ԭ����������д���ӷ���ʽ��
__________________________________________________________________��
��3������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ���� ���� ��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������¡�
a����Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ����Ĥ�������ö��Ե缫��⡣
b��������LiOH��Һ�м������NH4HCO3��Һ�����ˡ���ɵøߴ�Li2CO3��
�� a�У������ĵ缫��Ӧʽ��______________________________________��
�� b�У�����Li2CO3��Ӧ�Ļ�ѧ����ʽ��______________________________��