��Ŀ����
��ˮ�Ǿ����Դ���⣬�ں�ˮ�������ۺ����÷��棬�����λ��ȫ��ǰ�С��Ӻ�ˮ����ȡʳ�κ���Ĺ�������ͼ��
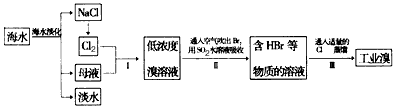
(1)����I���ѻ��Br2����������ֽ�Br2��ԭΪBr-����Ŀ��Ϊ____________________��
(2)�������SO2ˮ��Һ����Br2�������ʿɴ�95%����Ӧ����ʽΪ__________________��
(3)Br-�ļ�����Ҫ�Լ���__________�����ӷ���ʽΪ_______________________��
(4)�ӹ�ҵ�����ᴿ�壬�������й����ϣ�Br2����ɫΪ__________ ���е�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԣ�
(5)������ҵ����Һ��������Cl2���������м���NaBr��Һ����Ӧ�����ӷ���ʽΪ_________________����Ӧ������������________��
(2)�������SO2ˮ��Һ����Br2�������ʿɴ�95%����Ӧ����ʽΪ__________________��
(3)Br-�ļ�����Ҫ�Լ���__________�����ӷ���ʽΪ_______________________��
(4)�ӹ�ҵ�����ᴿ�壬�������й����ϣ�Br2����ɫΪ__________ ���е�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԣ�
(5)������ҵ����Һ��������Cl2���������м���NaBr��Һ����Ӧ�����ӷ���ʽΪ_________________����Ӧ������������________��
(1)������Ԫ��
(2)Br2+SO2+2H2O = H2SO4+2HBr
(3)AgNO3��Ag++Br- = AgBr��
(4)�����ɫ
(5)Cl2+2Br- = Br2+2Cl-����Һ��������
(2)Br2+SO2+2H2O = H2SO4+2HBr
(3)AgNO3��Ag++Br- = AgBr��
(4)�����ɫ
(5)Cl2+2Br- = Br2+2Cl-����Һ��������

��ϰ��ϵ�д�
�����Ŀ
��ˮ�Ǿ����Դ���⣬���й��ں�ˮ�ۺ����õ�˵��������ǣ�������

| A����ˮ�Ƶ�ˮ��Ҫ�������������������ӽ������� | B����ˮ���Ρ���չ�ȼҵ���Ƿ��������仯 | C����ˮ�����������ͨ��Cl2������������Ϊ�嵥�� | D����ҵ���õ������MgCl2�ķ�����ȡ����þ |




