��Ŀ����
��ʵ��������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ�������Ϊ�˼���SO2��
C2H4�����ʣ������������ʵ�鷽����
C2H4�����ʣ������������ʵ�鷽����

��1��I��II��III��IVװ�ÿ�ʢ�ŵ��Լ���I ________��II________��III________��IV________���������й��Լ����������ո��ڣ���
A��Ʒ����Һ B��NaOH��Һ C��ŨH2SO4 D������KMnO4��Һ
��2����˵��SO2������ڵ�������______________________��
��3��ʹ��װ��II��Ŀ����________________________��
��4��ȷ��������ϩ��������_____________________________��
��5�������ʢ�ŵ���ҺΪ��ˮ����ô�����Ļ�ѧ����ʽΪ________________________����Ӧ����Ϊ
________________ ��
A��Ʒ����Һ B��NaOH��Һ C��ŨH2SO4 D������KMnO4��Һ
��2����˵��SO2������ڵ�������______________________��
��3��ʹ��װ��II��Ŀ����________________________��
��4��ȷ��������ϩ��������_____________________________��
��5�������ʢ�ŵ���ҺΪ��ˮ����ô�����Ļ�ѧ����ʽΪ________________________����Ӧ����Ϊ
________________ ��
��1��A��B��A��D
��2��װ��I��Ʒ����ɫ
��3����ȥ�����������������ϩ�ļ���
��4��װ��III��Ʒ�첻��ɫ��װ��IV��KMnO4��Һ��ɫ
��5��CH2=CH2+Br2��BrCH2-CH2Br���ӳɷ�Ӧ
��2��װ��I��Ʒ����ɫ
��3����ȥ�����������������ϩ�ļ���
��4��װ��III��Ʒ�첻��ɫ��װ��IV��KMnO4��Һ��ɫ
��5��CH2=CH2+Br2��BrCH2-CH2Br���ӳɷ�Ӧ

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
 ��ʵ��������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ������������������ʵ����ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
��ʵ��������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ������������������ʵ����ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
 ��ʵ��������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ����������������ͼʵ����ȷ�����������������C2H4��SO2��
��ʵ��������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ����������������ͼʵ����ȷ�����������������C2H4��SO2��
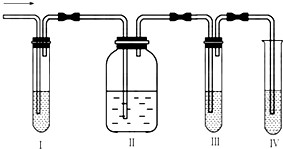 ��ʵ��������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ������������������ʵ��ͼ��ȷ�����������������C2H4��SO2��
��ʵ��������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ������������������ʵ��ͼ��ȷ�����������������C2H4��SO2��