��Ŀ����
(1)�ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣��ҵ�ϳɰ���ԭ���ǣ�N2+3H2
��X�Ļ�ѧʽΪ______________��
����ͼ������ѡ������Ҫԭ����(ѡ����ĸ���)__________��
A.�����¶ȡ�����ѹǿ�������ڰ��ĺϳ�
B.����ý�ڸ��¶�ʱ���Դ�
C.��ҵ�����ܶ��������ϡ��豸������������
�۸ı䷴Ӧ��������ʹƽ�ⷢ���ƶ�����ͼ��ʾ�������ı䣬�����İٷֺ����ı仯���ơ���������Ϊѹǿʱ���仯������ȷ����(ѡ����ĸ���)___________����������Ϊ�¶�ʱ���仯������ȷ����(ѡ����ĸ���)______________��

(2)�����°�����������ˮ����ˮ��Һ���Ե��硣
���÷���ʽ��ʾ��������ˮ���������ԵĹ��̣�____________________________________��
�ݰ�ˮ��ˮ�������c(OH-)_________10-7 mol��L-1(��д����������������)��
����ͬ�������ͬ���ʵ���Ũ�ȵİ�ˮ�������Ϻ���Һ������Ũ���ɴ�С����Ϊ____________��
(1)��NH3 ��BC ��c a
(2)��NH3+H2O![]() NH3��H2O
NH3��H2O![]()
![]() +OH- �ݣ� ��c(Cl-)��c(
+OH- �ݣ� ��c(Cl-)��c(![]() )�� c(H+)��c(OH-)
)�� c(H+)��c(OH-)
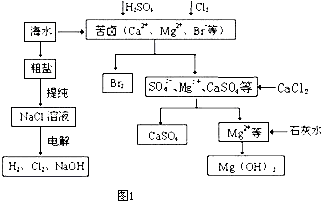
������(1)�������Ӽ�����������Һ������XΪ��������Ӧ������ѡ�����¶�Ӧ�Դ����Ļ�����ǿΪǰ�ᣬѹǿӦ�Բ��ϡ��豸�ij�������Ϊǰ�ᡣѹǿ������ƽ�������ƶ��������ĺ������࣬��ѡc���¶����ߣ�����ƽ�������ƶ��������ĺ������ͣ���ѡa��
(2)��������ˮ���ɰ�ˮ����ˮ�ܷ������룬����ˮ����̶ȼ�С���Ȼ���е�笠�������ˮ�⣬������Һ�����ԡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���12�֣��������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A�����ںϳɰ��Ĺ�ҵú���к���H2S��C2H5SH�����ᴼ����COS���ʻ���CS2�Ⱥ������ҵ������������п���������л������������������
H2S+ZnO=ZnS+H2O��C2H5SH+ZnO=ZnS+C2H4+H2O
C2H5SH+H2=C2H6+H2S��COS+H2=CO+H2S;CS2+4H2=CH4+2H2S
��1����ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2�������йط��ӽṹ��˵����ȷ���� ��
A��C2H4��������5�� ����1��
����1��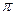 ��
��
B��COS���ӣ��ṹ����ͼ���м���C=O>C=S
C��H2S���ӳ�V�νṹ
D��CH4��C2H6������̼ԭ�Ӿ�����sp3�ӻ�
��3�������й�˵������ȷ���� ��
A��H2O��CO��COS���Ǽ��Է���
B����ͬѹǿ�·е㣺Cs2>COS >CO2
>CO2
C����ͬѹǿ�·е㣺C2H 5SH>C2H5OH
5SH>C2H5OH
D����ͬѹǿ�·е㣺CO>N2
��4�� -ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
-ZnS�ľ����ṹ����ͼ��������S2-��ĿΪ�� ����
��5���������ƾ����ṹ��ZnS��ZnO��ZnS�۵�Ϊ1830�棬ZnO�۵�Ϊ1975�棬���߽�ǰ�߸������� ��
��6�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]��8H2O������ԭ�ӵ���λ��Ϊ ��
B����ȩ��Ϸ�Ӧ�л��ϳ�����Ϊ��Ҫ����ɫ�����Ĺ��������ᱶ���о��߹�ע��������нϸߵĴ����Լ��ȶ��ԡ���Ӧԭ�����£�
ʵ�鷽������25mL��ƿ�м������ᡢ10mL�״��� 0.5mL����ȩ���ڻ���״̬�·�Ӧ2h����Ӧ�IJ��ʺ�ת���ʾ��dz��ߡ�
��1�����û�����Ӧ2h��Ŀ���� ��
��2���ڷ�Ӧ�м״����������ԭ���� �� ��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
��3����ͬ���������Բ��ʺ�ת����Ӱ�죬���±���
| ��������/mol | 0.01 | 0.02 | 0.03 | 0.05 | 0.1 | 0.15 | 0.2 | 0.6 |
| ����% | 87.3 | 88.2 | 90.3 | 94.2 | 92.9 | 93.1 | 91.8 | 92.3 |
| ת����% | 89.7 | 92.1 | 93.9 | 98.9 | 94.9 | 95.7 | 93.9 | 94.3 |
��4�������Ļ������������ǿ��������һ�Ϊ��Ҫ��ָ�ꡣ�������ѭ��ʹ�ô����Բ��ʵ�Ӱ��������ͼ����˵������������ŵ�֮һ�� ��
��5��������������ʱ����ͬ��ȩ��״������Ϸ�Ӧ��ת���ʺͲ������±���
| ��� | ȩ | �� | ת����% | ����% |
| 1 | ���ǻ�����ȩ | �״� | 94.3 | 89.6 |
| 2 | ���ǻ�����ȩ | �״� | 93.6 | 88.7 |
| 3 | ���ȱ���ȩ | �״� | 93.1 | 87.3 |
| 4 | ����������ȩ | �״� | 54.2 | 34.1 |
| 5 | ����������ȩ | �״� | 89.9 | 79.5 |
| 6 | ����������ȩ | �״� | 65.7 | 41.9 |

�ӱ��еó��IJ�ͬ��ȩ��״����Ϸ�ӦӰ��ת���ʺͲ��ʵĹ����� ��


 ��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣
��1���ϳɰ���ҵ�Ի�ѧ��ҵ������ҵ������Ҫ���塣