��Ŀ����
����A��B��C��������������������A��B��C������H��O��S��Na�е�����Ԫ����ɵ�ǿ����ʣ���A��B��ˮ��Һ���ʼ��ԣ�����AΪ�BΪ���Σ���C��ˮ��Һ�����ԣ������й�˵����ȷ���ǣ�������
| A��C��һ����H2SO4 |
| B�����ʵ���Ũ����ȵ�A��B����Һ��pH��С��ϵ��ǰ��С�ں��� |
| C��B������ˮ�п����ɳ��� |
| D����A��B����Һ��pH=9��������Һ����ˮ�������OH-�����ʵ���Ũ��֮��Ϊ1��104 |
���㣺����ˮ���Ӧ��
ר�⣺�����ˮ��ר��
������A��B��C�������ʶ�����H��O��S��Na�е�����Ԫ����ɵ�ǿ����ʣ����ݳ��������������A��B��ˮ��Һ���ʼ��ԣ������ж�ΪNaOH��ˮ�����Na2SO3��NaHS��AΪ�BΪ���Σ�AΪNaOH��BΪ��Na2SO3��C��ˮ��Һ�����Կ���ΪH2SO4��
A���ж�CΪ���
B�����������ǼBΪ��ˮ���Լ��ԣ�
C���嵥�ʾ��������ԣ�����B���������Σ�
D��ˮȫ��������������ӻ����������ӣ����ˮ�������������Ũ�ȣ�������Һ�����ӻ��������㣮
A���ж�CΪ���
B�����������ǼBΪ��ˮ���Լ��ԣ�
C���嵥�ʾ��������ԣ�����B���������Σ�
D��ˮȫ��������������ӻ����������ӣ����ˮ�������������Ũ�ȣ�������Һ�����ӻ��������㣮
���
�⣺�Լ��Ե����ʿ�����NaOH��Na2SO3��NaHS��˵��A��NaOH��BΪ������Na2SO3��C��ˮ��Һ��������Ϊǿ����ʣ�ӦΪ�����BΪNa2SO3������ˮ������Ӧ�����������ƣ�AΪNaOH��ˮ�����OH-����Һ��H+Ũ����ͬ��Ϊ10-9 mol/L��BΪ��ˮ����Σ���Һ�е�OH-ȫ����ˮ���룬��Ũ��Ϊ109-14 mol/L���ʶ���֮��Ϊ1��104��
A��C��H2SO4����A����
B�����ʵ���Ũ����ȵ�A��B����Һ��A��NaOH��B��Na2SO3����Һ��pH��С��ϵA����B����B����
C����BΪNa2SO3������ˮ������Ӧ��SO32-+H2O+Br2�TSO42-+2H++2Br-������������S��������C����
D��AΪNaOH��ˮ�����OH-����Һ��H+Ũ����ͬ��Ϊ10-9 mol/L��BΪ��ˮ����Σ���Һ�е�OH-ȫ����ˮ���룬��Ũ��Ϊ10-5mol/L���ʶ���֮��Ϊ1��104����D��ȷ��
��ѡD��
A��C��H2SO4����A����
B�����ʵ���Ũ����ȵ�A��B����Һ��A��NaOH��B��Na2SO3����Һ��pH��С��ϵA����B����B����
C����BΪNa2SO3������ˮ������Ӧ��SO32-+H2O+Br2�TSO42-+2H++2Br-������������S��������C����
D��AΪNaOH��ˮ�����OH-����Һ��H+Ũ����ͬ��Ϊ10-9 mol/L��BΪ��ˮ����Σ���Һ�е�OH-ȫ����ˮ���룬��Ũ��Ϊ10-5mol/L���ʶ���֮��Ϊ1��104����D��ȷ��
��ѡD��
���������⿼���������ƶϷ������������ʵ�Ӧ�ã���Һ����Եķ����ƶϣ�ˮ�ĵ�������е����ӻ�����Ӧ�ã���Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
���и���Ӧ�����ڼӳɷ�Ӧ���ǣ�������
A��CH2�TCH2+H-OH
| |||
B��H2+Cl2
| |||
C��2CH3-CH2-OH+O2
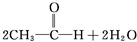 | |||
D��CH3-CH3+2Cl2
|
��֪A��B��C��D��ԭ��������������18�����ǵ�����aA��n+1��+��bBn+��cC��n+1��-��dDn-������ͬ�ĵ��Ӳ�ṹ��������˵����ȷ���ǣ�������
| A��ԭ��������a��b��c��d |
| B�����Ӱ뾶��A��n+1��+��Bn+��C��n+1��-��Dn- |
| C�����������ԣ�A��n+1��+��Bn+���ӻ�ԭ�ԣ�C��n+1��-��Dn- |
| D�����ʻ�ԭ�ԣ�B��A ���������ԣ�D��C |
����˵��������ǣ�������
| A��ԭ������������Ϊ2��Ԫ��һ������Ԫ�����ڱ��ڢ�A�� |
| B��L�������Ϊ����������Ԫ����������������Ԫ��ԭ�ӵ�L���������ͬ |
| C������������Ԫ��X��Y���γ�XY2�ͻ������X��Y ��ԭ������֮�����Ϊ1��2 |
| D��M�������Ϊ��������������Ԫ����������������Ԫ��ԭ�ӵ�M���������ͬ |
��֪�ס��ҡ�������������Һ��ΪAlCl3��NH3?H2O��CH3COOH��NaCl�е�һ�֣���ͬ�¶��£���������Һ��pH��ͬ���ͱ���ˮ�ĵ���̶���ͬ������ǣ�������
| A��NH3?H2O |
| B��AlCl3 |
| C��CH3COOH |
| D��NaCl |
 ����V�����仯����㷺Ӧ���ڹ�ҵ�����²��Ϻ�����Դ������
����V�����仯����㷺Ӧ���ڹ�ҵ�����²��Ϻ�����Դ������